PRESS RELEASE: Fifth International Pharmaceutical Compliance Congress Features Co chair Dominique Laymand, Esq., Executive Director Compliance & Ethics EMEA (Europe, Middle-East, Africa, Russia and Turkey), Bristol-Myers Squibb

- Sponsored by the Pharmaceutical Compliance Forum
- Featuring Tracks on EU, CEE and MEA
- May 3 - 5, 2011
- Renaissance Polat Istanbul Hotel
- Istanbul, Turkey
- www.InternationalPharmaCongress.com
PRESS RELEASE
Phone: 800-684-4549
Email: registration@hcconferences.com
Website: www.InternationalPharmaCongress.com
WASHINGTON DC USA -- PHARMA UPDATE NEWS SERVICE™ -- FEBRUARY 24, 2011:
The International Pharmaceutical Compliance Congress, www.InternationalPharmaCongress.com, May 3 - 5, 2011 will be held at the Renaissance Polat Istanbul Hotel in Istanbul, Turkey. The International Pharma Congress is sponsored by the Pharmaceutical Compliance Forum.
|
FEATURING CONGRESS CO CHAIR DOMINIQUE LAYMAND, ESQ.
|
|
 Dominique Laymand is Executive Director Compliance & Ethics EMEA (Europe, Middle East, Turkey, Russia and Africa). In this role, Dominique is responsible for maintaining, monitoring and controlling a compliance & ethics model that proactively addresses key compliance matters for EMEA. She works closely with the EMEA Business leadership teams to establish policy and oversight of these areas. She has a particular focus on Business Practices and is a member of the Bristol-Myers Squibb Corporate Compliance Council and European Operating Committee.
Dominique Laymand is Executive Director Compliance & Ethics EMEA (Europe, Middle East, Turkey, Russia and Africa). In this role, Dominique is responsible for maintaining, monitoring and controlling a compliance & ethics model that proactively addresses key compliance matters for EMEA. She works closely with the EMEA Business leadership teams to establish policy and oversight of these areas. She has a particular focus on Business Practices and is a member of the Bristol-Myers Squibb Corporate Compliance Council and European Operating Committee.
Dominique has a broad experience at Bristol-Myers Squibb, having started with the company in 1983 as Legal Counsel. Since then, she has held increasingly responsible legal positions which have given her experience in various functions and units including Marketing, Medical, Pricing & Reimbursement, Human Resources and all the matters related to pharmaceutical laws.
Dominique received her Business Law Degree from Montpellier University in 1983 and her Health Law Degree in Sceaux University in 1996.
|
|
|
|
KEYNOTE SPEAKER
|

David Brennan
Chief Executive Officer, AstraZeneca, President, International Federation of Pharmaceutical, Manufacturers and Associations (IFPMA), Past Chairman, Pharmaceutical Research, and Manufacturers of America (PhRMA), London, UK
|
|
|
|
|
|
WELCOME ADDRESS
|

Prof. Dr. Cevdet Erdöl
Parliament Member and Chairman, Health, Family, Work & Social Works Commission, The Grand National Assembly of Turkey, Ankara, Turkey
|
|
|
|
|
PHARMA COMPLIANCE TRACKS
|
- European Union Compliance Update
- Central and Eastern Europe Compliance Update
- Middle East and Africa Compliance Update
- FCPA and Global Anticorruption
|
- Global Compliance Audits and Monitoring
- Global Transparency, Discourse and Aggregate Spend
- Global Compliance Case Studies
|
|
OTHER KEYNOTE FACULTY
|

Philippa Foster Back, OBE
Director, Institute of Business Ethics, Chairman, UK Antarctic Heritage Trust, Past President, Association of Corporate Treasurers, London, UK |

Bulent Becan
Managing Partner, Atuva Management Consultancy and Trade Ltd., Consultant in Ethics Issues, Turkish Research Based Pharmaceutical Companies Association (AIFD), Istanbul, Turkey |

Joe Henein
President and Chief Executive Officer, NewBridge Pharmaceuticals, Former Regional Managing Director, Middle East & North Africa, Wyeth Pharmaceuticals, Dubai, United Arab Emirates
|

Jose F. Zamarriego Izquierdo
Director Unidad de Supervision Deontologica, FARMAINDUSTRIA, Madrid, Spain |

Philippe Montigny
Certification Committee President, ETHIC Intelligence International, President, ETHIC Intelligence France, Executive Director and Partner, International Development & Strategies - France, Paris, France |

Chrisoula Nikidis
Director, Ethics and Compliance, Canada's Research-Based Pharmaceutical Companies (Rx&D), Ottawa, ON, Canada
|

Marie-Claire Pickaert
Deputy Director General, European Federation of Pharmaceutical Industries and Associations (EFPIA), Brussels, Belgium |

Mark Pieth, PhD
Professor of Criminal Law, Basel University, Chairman, Working Group on Bribery in International Business Transactions, Organization for Economic Co-operation and Development (OECD), Member, Swiss Federal Gaming Commission, Board Chairman, Basel Institute on Governance, Basel, Switzerland
|

Vladimir Shipkov
Chief Executive Officer, Association of International Pharmaceutical Manufacturers, Member, IFPMA Council, Former Head, Ministry of Health Pharmaceutical Inspectorate, Former Deputy Chief, Federal Service for Intellectual Property, Patents and Trademarks (Rospatent), Moscow, Russian Federation |

Heather Simmonds
Director and Chair, Code of Practice Panel, Prescription Medicines Code of Practice Authority, London, UK
|
|
|
|
SPECIAL SESSIONS ON EUROPEAN UNION COMPLIANCE ISSUES BY:
|

Ted Acosta, Esq.
Principal, Ernst & Young LLP, Former Senior Counsel, Office of Inspector General, US Department of Health and Human Services, New York, NY, USA and Paris, France |

Gabor Danielfy
Senior Director, Health Care Compliance and Privacy EMEA, Johnson & Johnson, Issy-les-Moulineaux, France |

Pierre E. Dupourque
Regional Compliance Director, Corporate Compliance, International Investigations and Programs, Pfizer Inc, Mannheim, Germany
|

Sylvia Fondaneche
Director, International Congresses & Events, sanofi-aventis, President, International Pharmaceutical, Congress Advisory Association (IPCAA), Paris, France |

Gary F. Giampetruzzi, Esq.
Vice President and Assistant General Counsel, Head of Government Investigations, Pfizer Inc, New York, NY, USA |

Thomas Kellner
Global Academic and Professional Affairs, Merck & Co., Inc., Board of Directors, Global Alliance for Medical Education, Munich, Germany
|

Bernard Maillet, MD
Secretary General, European Union of Medical Specialties (UEMS) And European Accreditation Council for Continuing Medical Education (EACCME), Brussels, Belgium |

Brian Riewerts
Partner, Global Pharmaceuticals and Life Sciences, PricewaterhouseCoopers LLP, Baltimore, MD, USA
|

Marco Sugarelli, Esq.
Legal Affairs Associate Director, Pfizer Italia, Rome, Italy |

Paul B. Woods, BPharm, MA, MRPharmS
Global Compliance Policy Director, AstraZeneca, Macclesfield, Cheshire, UK
|
|
|
SPECIAL SESSIONS ON CENTRAL AND EASTERN EUROPEAN
COMPLIANCE ISSUES BY:
|

Olga Kozyr, Esq.
Partner, Hogan Lovells International LLP, Moscow, Russian Federation |

Artur Nagapetyan
Country Compliance Officer, Novartis Pharma LLC, Moscow, Russian Federation |

Martin Schloh, PhD
Partner, Global Pharmaceuticals and Life Sciences, PricewaterhouseCoopers LLP, Munich, Germany
|

Kersten Schmahl, Esq.
Visiting Lecturer Health Care & FCPA-Compliance, Business Law Institute, Leuphana University, Zug, Switzerland |

Madina Torchinova, Esq.
Director Legal and Regulatory Affairs, Association of International Pharmaceutical Manufacturers (AIPM), Moscow, Russian Federation |

Mariusz Witalis
Partner, Fraud Investigation and Dispute Services, Ernst & Young LLP, Warsaw, Poland
|
|
|
SPECIAL SESSIONS ON MIDDLE EAST/AFRICA COMPLIANCE ISSUES BY:
|

Nabil Daoud
Managing Director, MENA, Eli Lilly and Company, Chair, MEA LAWG Ethics Review Board, Beirut, Lebanon |

Nidal Fakhoury
Regional Managing Director - Middle East, MSD, Merck, Chairman, PhRMAG, Dubai, United Arab Emirates |

Sameh Farag
Regional Compliance Director, MEA, Merck, Dubai, United Arab Emirates
|

Liz MacGillivray
Head of Compliance AMAC Region (Asia Pacific, Middle East & African Countries), Novartis, Jumeirah Village, United Arab Emirates |

Kirti Narsai, MSc(Pharm), MBA
Head: Scientific and Regulatory Affairs, Pharmaceutical Industry Association of South Africa, Vorna Valley, South Africa |

Yasser A. Omar, LL.B.
Partner, Shalakany Law Firm, Dubai, United Arab Emirates
|
|
|
SPECIAL SESSIONS ON GLOBAL COMPLIANCE AUDITING AND MONITORING BY:
|

Bret A. Campbell, Esq.
Partner, Business Fraud and Complex Litigation Practice, Cadwalader, Wickersham & Taft LLP, Washington, DC, USA |

Peter Dieners, Esq.
Partner, Clifford Chance, Düsseldorf, Germany |

Patricia A. Etzold, CPA
Partner, PricewaterhouseCoopers LLP, New York, NY, USA
|

Barbara R. Giddings, RN, MHA, CHC
Global Commercial Compliance, Biogen Idec, Weston, MA, USA |

Ryan Murphy
Director, PricewaterhouseCoopers LLP, Chicago, IL, USA
|
|
|
SPECIAL SESSIONS ON FCPA AND ANTICORRUPTION BY:
|

John T. Bentivoglio, Esq.
Partner, Skadden Arps LLP, Former Special Counsel for Healthcare Fraud, and Chief Privacy Officer, Department of Justice, Washington, DC, USA |

Jeremy Cole, Esq.
Partner, Hogan Lovells, London, UK |

Gary DiBianco, Esq.
Partner, Skadden Arps LLP, Former Trial Attorney, Criminal Division, Former Special Assistant US Attorney, Eastern District of Virginia, US Department of Justice, London, UK
|
John Parsons
Associate Director, Head of EUMEA Legal and Compliance, BioMarin Europe Limited, London, UK |

SanDee Priser
Partner, Fraud Investigation and Dispute Services, Ernst & Young LLP, Frankfurt am Main, Germany |

Michael Roberts, Esq.
Senior Associate, Hogan Lovells, London, UK
|

Jeffrey Rosenbaum
Global Head, Ethics and Compliance, Novartis Oncology, Florham Park, NJ, USA |

Joseph B. Tompkins, Jr.
Partner, Sidley Austin LLP, Former Deputy Chief of the Fraud Section, Criminal Division of the United States Department of Justice, Washington, DC, USA |

John Wilson
Global Lead - AntiBribery and AntiCorruption Programme, AstraZeneca, London, UK
|
|
|
SPECIAL SESSIONS ON GLOBAL TRANSPARENCY, DISCLOSURE AND AGGREGATE SPEND ISSUES BY:
|

Ronny Arijs
Vice President Global Sustainability & Chief Compliance Officer, Grunenthal, Brussels, Belgium |

Bridget Bourgeois
Partner, Ernst & Young LLC, Atlanta, GA, USA
|

Michael K. Loucks, Esq.
Partner, Skadden Arps LLP, Former Acting United States Attorney, US Attorney's Office for the District of Massachusetts, Boston, MA, USA |

Guillaume Roussel
Vice President, Compliance Solutions EMEA, Cegedim, Paris, France
|
|
|
SPECIAL GLOBAL COMPLIANCE CASE STUDIES BY:
|

Sue Egan
Director and Principal Consultant, Sue Egan Associates, Former Vice President Compliance, AstraZeneca, London, UK |

Keith M. Korenchuk, JD, MPH
Partner, Arnold & Porter LLP, Washington, DC, USA
|

Maxine Nogard
Senior Director, Global Corporate Compliance, Biogen Idec Inc., Weston, MA, USA
|

Elisabethann Wright, Esq.
Partner, Hogan Lovells, Former, Senior Legal Officer and Hearing Officer, EFTA Surveillance Authority, Brussels, Belgium
|
|
|
A SPECIAL REPORT FROM ASIA BY:
|

John Auerbach, MA
Partner, Fraud Investigation & Dispute Services, Ernst & Young China, Shanghai, China |

Yuet-Ming Tham, Esq.
Partner and Head, Regulatory, Compliance & Investigations Group, AsiaDLA Piper, Former Regional Compliance Director, Legal Division, Corporate Compliance, Pfizer Inc, Hong Kong
|
|
|
AND A SPECIAL SESSION ON THE FUTURE: ENVISIONING PHARMA COMPLIANCE IN 2015
|

Michael H. Friedland, Esq.
Deputy Compliance Officer, Internal Investigations and Assistant General Counsel, Pfizer Inc, New York, NY, USA |

Stephen F. Mohr, Esq.
Global Compliance Officer, AstraZeneca, Wilmington, DE, USA
|

Sheila E. Stranks, MBA
Vice President and Deputy Compliance Officer, Shire Pharmaceuticals Group PLC, Hampshire, UK |

Brian Riewerts
Partner, Global Pharmaceuticals and Life Sciences, PricewaterhouseCoopers LLP, Baltimore, MD, USA (Moderator)
|
|
|
|
|
|
|
|
EARLY BIRD REGISTRATION
|
Click Here to register by Friday, March 25, 2011 and save €100.
|
|
|
BROCHURE AVAILABLE
|
Click here to view the brochure.
|
|
|
SPONSORED BY:
|
|

The Pharmaceutical Compliance Forum (PCF) is a coalition of senior compliance professionals and legal counsel from more than 50 of the largest research-based pharmaceutical manufacturers. The PCF was founded in early-1999 by compliance professionals from the pharmaceutical industry to promote effective corporate compliance programs. The members meet twice a year, for two days, focusing on open and informal sharing of compliance information, best practices, and current developments in the field, and sponsors a two-day international compliance congress in the Spring and a three-day US compliance congress each Fall.
|
|
|
CONGRESS CO CHAIRS
|

Kelly B. Freeman, PhD
Ethics and Compliance Officer, Eli Lilly and Company, Executive Committee Member, Pharmaceutical Compliance Forum, Indianapolis, IN, USA
|

Dominique Laymand, Esq.
Executive Director Compliance & Ethics EMEA (Europe, Middle-East, Africa, Russia and Turkey), Bristol-Myers Squibb, Paris, France
|

Roeland Van Aelst
Vice President EMEA & Canada, J&J Office Health Care Compliance & Privacy, Johnson&Johnson, Brussels, Belgium
|
|
|
GRANTORS:
|
|
DIAMOND
|

|
|
GOLD
|


|
|
BRONZE
|





|
|
2011 INTERNATIONAL PHARMA CONGRESS PLANNING COMMITTEE
|
Kelly B. Freeman, PhD
Ethics and Compliance Officer, Eli Lilly and Company, Indianapolis, IN, USA (Co chair)
Dominique Laymand, Esq.
Executive Director Compliance & Ethics EMEA (Europe, Middle-East, Africa, Russia and Turkey), Bristol-Myers Squibb, Paris, France (Co chair)
Roeland Van Aelst
Vice President EMEA & Canada, J&J Office Health Care Compliance & Privacy, Johnson&Johnson, Brussels, Belgium (Co chair)
Ted Acosta, Esq.
Principal, Ernst & Young LLP, Former Senior Counsel, Office of Inspector General, US Department of Health and Human Services, New York, NY, USA and Paris, France
Ann Beasley Bacon
Global Compliance Officer, Novartis Pharma AG, Basel, Switzerland
Wayne Baker
Sr. Vice President, Advanced Health Media LLC, Bridgewater, NJ, USA
Karie Jo Barwind, Esq.
Area Director Business Practices, Western Europe, Abbott Laboratories, Rungis Cedex, France
Bulent Becan
Managing Partner, Atuva Management Consultancy and Trade Ltd., Consultant in Ethics Issues, Turkish Research Based Pharmaceutical Companies Association (AIFD), Istanbul, Turkey
John T. Bentivoglio, Esq.
Partner, Skadden Arps LLP, Washington, DC, USA
Gabor Danielfy
Senior Director, Health Care Compliance and Privacy EMEA, Johnson & Johnson, Issy-les-Moulineaux, France
Pierre E. Dupourque
Regional Compliance Director, Corporate Compliance, International Investigations and Programs, Pfizer Inc, Mannheim, Germany
Sue Egan
Director and Principal Consultant, Sue Egan Associates, Former Vice President Compliance, AstraZeneca, Great Missenden, Buckinghamshire, UK
Sameh Farag
Regional Compliance Director, MEA, Merck, Dubai, United Arab Emirates
Gerard Geneen
Vice President, Compliance Officer, Pharma Europe, GlaxoSmithKline, Brentford, Middlesex, UK
Gary F. Giampetruzzi, Esq.
Vice President and Assistant General Counsel, Head of Government Investigations, Pfizer Inc, New York, NY, USA
Keith M. Korenchuk, JD, MPH
Partner, Arnold & Porter LLP, Washington, DC, USA
Maxine Nogard
Senior Director, Global Corporate Compliance, Biogen Idec Inc., Weston, MA, USA
Brian Riewerts
Partner, Global Pharmaceuticals and Life Sciences, PricewaterhouseCoopers LLP, Baltimore, MD, USA
Jeffrey Rosenbaum
Global Head, Ethics & Compliance, Novartis Oncology, Florham Park, NJ, USA
Guillaume Roussel
Vice President, Compliance Solutions EMEA, Cegedim, Paris, France
Joseph B. Tompkins, Jr.
Partner, Sidley Austin LLP, Former Deputy Chief of the Fraud Section, Criminal Division of the United States Department of Justice, Washington, DC, USA
Paul B. Woods, BPharm, MA, MRPharmS
Global Compliance Policy Director, AstraZeneca, Macclesfield, Cheshire, UK
Elisabethann Wright, Esq.
Partner, Hogan Lovells, Former, Senior Legal Officer and Hearing Officer, EFTA Surveillance Authority, Brussels, Belgium
|
|
|
PAST INTERNATIONAL PHARMA CONGRESSES
|

2007 - Brussels, Belgium

2008 - Paris, France

2009 - Rome, Italy

2010 - Berlin, Germany
|
|
|
SAVE THE DATE
|
FIRST ASIAN PHARMACEUTICAL COMPLIANCE CONGRESS

Sponsored by Pharmaceutical Compliance Forum
September 13 - 15, 2011
The Ritz-Carlton, Millenia
Singapore
www.AsianPharmaCongress.com
|
TWELFTH PHARMACEUTICAL REGULATORY AND COMPLIANCE CONGRESS

Sponsored by Pharmaceutical Compliance Forum
November 2 - 4, 2011
Mandarin Oriental
Washington, DC
www.PharmaCongress.com
|
|
|
FOLLOW INTERNATIONAL PHARMA CONGRESS ON
|
|
|

|

|
|
NEW PARTICIPATION OPTION: LIVE AND ARCHIVED INTERNET ATTENDANCE
|
|
Announcing a new way to participate in the International Pharma Congress: You can now register to watch the Congress in live streaming video over the Internet and at your convenience at any time 24/7 for the six months following the event.
Click here to register.
|
|
|
TRADITIONAL ONSITE ATTENDANCE
|
|
|
|
|
LIVE AND ARCHIVED INTERNET ATTENDANCE
|
|
Watch the conference in live streaming video over the Internet and at your convenience
at any time 24/7 for the six months following the event.
The archived conference includes speaker videos and coordinated PowerPoint presentations.
Pros: Live digital feed and 24/7 Internet access for next six months; Accessible in office, at home or anywhere worldwide with Internet access; Avoid travel expense and hassle; No time away from the office
|
INTERNET INTERFACE SAMPLE
|
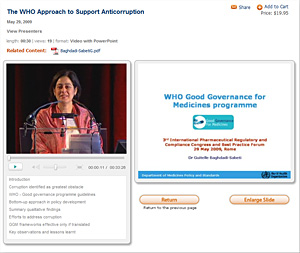
Click here for a sample stream
|
|
|
|
| CONGRESS EXHIBIT & SPONSORSHIP INFORMATION |
|
For sponsorship and exhibit information contact Joni Lipson, Exhibit Manager, 215-952-0866 phone, 215-952-0664 fax, joni.lipson@lipsonllc.com.
|
|
|
|





