|
|
|
Press Release: International Pharma Compliance Congress VIII Features Communicating with Health Care Professionals regarding Participation in Transparency Programs
|

- A Hybrid Conference and Internet Event
- Sponsored by ethics, the International Society of Healthcare Compliance Professionals
- Co-sponsored by the Pharmaceutical Compliance Forum
- May 5 - 7, 2014
- Onsite at the Conrad Dubai, Dubai, United Arab Emirates
- In Your Own Office or Home live via the Internet with 24/7 Access for Six Months
- www.InternationalPharmaCongress.com
PRESS RELEASE
Phone: 800-503-8171
Email: registration@hcconferences.com
Website: www.InternationalPharmaCongress.com
|
|
FEATURING COMMUNICATING WITH HCPS RE PARTICIPATION IN TRANSPARENCY PROGRAMS BY
|

Michel Ballieu
Chief Executive Officer, European CanCer Organisation, Brussels, Belgium |

Isabel Bardinet
Chief Executive Officer, European Society of Cardiology, Sophia Antipolis, France |

Richard Bergström
Director General, European Federation of Pharmaceutical Industries and Associations (EFPIA), Former Director General, LIF Sweden, Brussels, Belgium
|

Carin R. Smand, MSc
Managing Director, European Hematology Association, The Hague, The Netherlands |

Michael Bartke, PhD
Director Compliance Management, Daiichi Sankyo Europe GmbH, Co-chair, EFPIA Compliance Workgroup, Munich, Germany, (Moderator)
|
|
|
|
WASHINGTON DC USA -- CME UPDATE NEWS SERVICE™ -- JANUARY 28, 2014: The Eighth International Pharmaceutical Compliance Congress, www.InternationalPharmaCongress.com, will take place on May 5 - 7, 2014 at the Conrad Dubai in Dubai, United Arab Emirates. Registrants may attend the event in person or online (live webcast and archived for 6 months).

|
KEYNOTE SPEAKERS
|

Michel Ballieu
Chief Executive Officer, European CanCer Organisation, Brussels, Belgium |

Isabel Bardinet
Chief Executive Officer, European Society of Cardiology, Former Director and General Manager at SOCFI (Société d'Organisation de Congrès Français et Internationaux), Sophia Antipolis, France |

Richard Bergström
Director General, European Federation of the Pharmaceutical Industries and Associations (EFPIA), Former Director General, LIF Sweden, Brussels, Belgium
|

Holger Diener, PhD
Managing Director, Association of Voluntary Self-Regulation for the Pharmaceutical Industry, Berlin, Germany |

Dr. Yacoub Haddad
Chair, Pharmaceutical Research and Manufacturers Association, Gulf (PhRMAG), Government Affairs Director MEAP Region, Abbvie, Beirut, Lebanon |

Arthur Muratyan, Esq.
Secretary General, International Society of HealthCare Ethics and Compliance Professionals (ETHICS), Former Vice President-Head of Legal Corporate, and Global Compliance Officer, Sanofi, Paris, France
|

Jean-Claude Najar, LLM
Member, Curtis, Mallet-Prevost, Colt & Mosle LLP, Lecturer, Sciences Po Law School and HEAD Law School, Former General Counsel EMEA, GE Medical Systems (now GE Healthcare), Paris, France |

Prof. Dr. iur. Mark Pieth
Professor of Criminal Law and Criminology, University of Basel, Former Head of Section, Economic and Organised Crime, Swiss Federal Office of Justice, Former Chair, OECD Working Group on Bribery in International Business Transactions, Basel, Switzerland |

Christine Sainvil, Esq.
Compliance Officer, Eucomed, Brussels, Belgium
|

Heather Simmonds
Director and Chair, Code of Practice Panel, Prescription Medicines Code of Practice Authority, London, UK |

Carin R. Smand, MSc
Managing Director, European Hematology Association, The Hague, The Netherlands |

Jose F. Zamarriego Izquierdo
Director Unidad de Supervision Deontologica, FARMAINDUSTRIA, Madrid, Spain
|
|
|
THE CONGRESS FEATURES EU COMPLIANCE DEVELOPMENTS,
WITH SPECIAL REPORTS FROM CEE, MEA AND INDIA
|
|
|
FEATURING A FOCUS ON NEW PHARMA BUSINESS MODELS
AND RESULTING COMPLIANCE CHALLENGES
|
- Changing Business Models:
- Context:
- Rising Costs
- Decline of Blockbuster Model
- Rise of Specialty Care/Biologics
- Industry Trends:
- Consolidation
- Patent Cliff
- Mature Markets
- Growth of Emerging Markets
- Diversification into Diagnostics and Devices, Generics and Consumer Health
- More Partnerships, Collaborations, and Licensing Agreements
- Old Model Centered on Company/HCP Relationship
- New Model in US, ACA, Increased Governmental Funding and Negotiations with Insurers
- New Model Globally, Negotiation with Government
- Emerging of Comparative Effectiveness Research
- New Ethics and Compliance Issues Arise around:
- Market Access, Affordability, Infrastructure and Knowledge in Emerging Markets, Limiting Health Care Systems
- Managing Third Party Relationships, Increased Risk, Less Transparency
- Pricing and Reimbursement; Conditional upon Post Marketing Confirmation of Effectiveness
- Tendering
- R&D; Clinical Trials; Publications
- Medical Education
- New National Anticorruption Initiatives
- Proliferation of Transparency Initiatives
- The Role of Compliance Codes
|
|
|
|
FEATURING IFPMA CODE WORKSHOP AND ETHICAL PROMOTION ROUNDTABLE
|
 The International Federation of Pharmaceutical Manufacturers & Associations (IFPMA) is offering an IFPMA CODE WORKSHOP: HANDS-ON COMPLIANCE TRIANING on Sunday, May 4, 2014 and a COMPLIANCE ROUNDTABLE on the morning of Monday, May 5, 2014.
The International Federation of Pharmaceutical Manufacturers & Associations (IFPMA) is offering an IFPMA CODE WORKSHOP: HANDS-ON COMPLIANCE TRIANING on Sunday, May 4, 2014 and a COMPLIANCE ROUNDTABLE on the morning of Monday, May 5, 2014.
|
 |
Tamara Music, Manager, Influenza Vaccines & Code Compliance, IFPMA, Geneva, Switzerland (Chair)
|
|
|
AND A MIDDLE EAST/AFRICA (MEA) REGIONAL COMPLIANCE ROUNDTABLE
|
|
Sponsored by International Federation of Pharmaceutical Manufacturers & Associations (IFPMA) and Pharmaceutical Research and Manufacturers Association, Gulf (PhRMAG)
|
 |
|
|
On the morning of Monday, May 5, 2014 the following will co-chair a MEA Regional Compliance Roundtable:
|
 |
Nabil Daoud, MA, Vice President and Area Director TMEA-CIS, Eli Lilly, Chair, MEA Regional Ethics Group (LERB), Dubai, United Arab Emirates
|
|
 |
Dr. Yacoub Haddad, Chair, Pharmaceutical Research and Manufacturers Association, Gulf (PhRMAG), Government Affairs Director MEAP Region, Abbvie, Beirut, Lebanon
|
|
|
FEATURED FACULTY
|

Ted Acosta, Esq.
Principal, Ernst & Young LLP, Former Senior Counsel, Office of Inspector General, US Department of Health and Human Services, New York, NY, USA and Paris, France |

Timothy S. Ayers, Esq.
Principal, Porzio, Bromberg & Newman, PC, Vice President, Porzio Life Sciences, Former Vice President and Chief Compliance Officer, Dendreon Corporation, Morristown, NJ, USA |

Hulya Baran
Director, Ethics and Compliance TMEA - CIS, Eli Lilly and Company, Istanbul, Turkey
|

Bulent Becan
Managing Partner, Atuva Management Consultancy and Trade Ltd., Consultant in Ethics Issues, Turkish Research Based Pharmaceutical Companies Association (AIFD), Istanbul, Turkey |

Carsten Blaesberg
Chief Consultant, Lif Denmark, The Danish Association of the Pharmaceutical Industry, Copenhagen, Denmark |

Karl Buch, Esq.
Assistant General Counsel, Pfizer, New York, NY, USA
|

Nabil Daoud, MA
Vice President and Area Director TMEA-CIS, Eli Lilly, Chair, MEA Regional Ethics Group (LERB), Dubai, United Arab Emirates |

Sue Egan
Director and Principal Consultant, Sue Egan Associates, Former Vice President Compliance, AstraZeneca, Great Missenden, Buckinghamshire, UK |

Saad Fahmy
Compliance Director, Middle East & Pakistan, Sanofi, Former Compliance Officer (Gulf, Saudi, Arabia & Developing Markets), Merck, Dubai, United Arab Emirates
|

Pascale Feghali
Ethics & Compliance Director, Middle East & Egypt, Eli Lilly, Beirut, Lebanon |

Gary F. Giampetruzzi, Esq.
Vice President and Assistant General Counsel, Head of Government Investigations, Pfizer Inc., New York, NY, USA |

Meltem Ozker Gunduz
Legal Affairs and Compliance Director, Business Area Near East, Novo Nordisk, Istanbul, Turkey
|

Thomas A. Gunning, Esq.
Senior Vice President and General Counsel, Prescription Medicines' Division, Merck KGaA, Darmstadt, Germany |

Joe Henein
President and Chief Executive Officer, NewBridge Pharmaceuticals, Former Regional Managing Director, Middle East, and North Africa, Wyeth Pharmaceuticals, Dubai, United Arab Emirates |

Jonathon Kellerman
Principal, Pharmaceutical and Life, Sciences Advisory Services, PwC LLP, Florham Park, NJ, USA
|

Swee Kheng Khor, MD
Associate Director, Office of Ethics & Compliance, Middle East & Pakistan, AbbVie BioPharmaceutical, GmBH, Dubai, United Arab Emirates |

Keith M. Korenchuk, JD, MPH
Partner, Arnold & Porter LLP, Washington, DC, USA |

Daniel A. Kracov, Esq.
Partner and Head, FDA and Healthcare Practice, Arnold & Porter, Washington, DC, USA
|

Michael K. Loucks, Esq.
Partner, Skadden Arps LLP, Former Acting United States Attorney, District of Massachusetts, Washington, DC, USA |

Abdul Luheshi, MBA
Vice President Health Care Compliance, Asia Pacific, Johnson & Johnson, Former Co-chair, Asia Pacific Pharma Compliance Congress, Singapore |

Rajiv Malhotra
Senior Manager Compliance, Biogen Idec, Former Ethics and Compliance Officer, Eli Lilly and Company (India) Pvt Ltd, Gurgaon, India
|

Paul J. Melling, Esq.
Founding Partner, Baker & McKenzie - CIS, Limited, Moscow, Russia |

Laura Nassar
Regional Pharma HCC Officer Emerging Markets, Johnson & Johnson, Beirut, Lebanon |
Minal Patel
Compliance Officer, Pharmaceuticals & Consumer, South Africa & Sub Saharan Africa, Johnson & Johnson, Johannesburg, South Africa
|

Dr. Gudula Petersen
Chief Compliance Officer Europe & Australia, Grunenthal, Dortmund, Germany |

Vivian Robinson, Esq.
Partner, McGuire Woods, Former General Counsel of the UK Serious Fraud Office, Former Head, QEB Hollis Whiteman Chambers, Recorder of the Crown Court and Treasurer of Inner Temple, London, UK |

Jeff Rosenbaum, MBA
Vice President, Chief Compliance Officer, Vertex Pharmaceuticals, Former Executive Director, Global Head of Ethics & Compliance Novartis Oncology, Boston, MA, USA
|

Christian-Claus Roth
Head of Congresses and Conventions, Novartis Pharma, Vice President, International Pharmaceutical Congress Advisory Association (IPCAA), Berne, Switzerland |

Joseph B. Tompkins, Jr.
Partner, Sidley Austin LLP, Former Deputy Chief of the Fraud Section, Criminal Division, United States Department of Justice, Washington, DC, USA |

Elisabethann Wright, Esq.
Partner, Hogan Lovells; Former Senior Legal Officer and Hearing Officer, EFTA Surveillance Authority, Brussels, Belgium
|
|
|
|
|
|
|
SPEAKER PRESENTATION PROPOSALS
|
Speaker/Presentation Proposals for the International Pharma Congress May Be Submitted Through Our Online Form
- Click Here -
|
|
|
|
|
SPONSORED BY:
|
|

|
|
CO-SPONSORED BY:
|
|

|
|
CONGRESS CO CHAIRS
|

Ann Beasley
Senior Vice President and Chief Compliance Officer, Biogen Idec, GmbH, Board Member and Co-chair, Strategic Committee, International Society of Healthcare, Ethics and Compliance Professionals (ethics), Zug, Switzerland
|

Dr. Michael Bartke
Director Compliance Management, DAIICHI SANKYO EUROPE GmbH, Co-chair, EFPIA Compliance Workgroup, Munich, Germany
|

Dominique Laymand, Esq.
Vice President Compliance & Ethics EMEA, (Europe, Middle-East, Africa, Russia and Turkey), Bristol-Myers Squibb, President, International Society of Healthcare, Ethics and Compliance Professionals (ethics), Paris, France
|

Roeland Van Aelst
Vice President EMEA & Canada, Johnson & Johnson, Board Member and Co-chair, Strategic Committee, International Society of Healthcare, Ethics and Compliance Professionals (ethics), Brussels, Belgium
|
|
|
|
GRANTORS:
|
|
GOLD
|

|
|
BRONZE
|








|
|
FEATURING VIDEO PRESENTATIONS FROM PAST CONGRESSES:
|
|
2013 Seventh International Pharma Congress Keynote Address by
|

Huguette Labelle, CC, PhD, LLD
Chair, Transparency International, Companion of the Order of Canada, Berlin, Germany
|
|
2013 Fourteenth International US Pharma Congress Keynote Address by
|
Brent L. Saunders, JD, MBA
Chief Executive Officer, Forest Laboratories, Former Chief Executive Officer, Bausch + Lomb, Rochester, NY
|
|
2012 Second Asia Pacific Pharma Congress Keynote Address by
|

Mark Mallon, MBA
Regional Vice President Asia Pacific and President China, AstraZeneca, Former Executive Vice-President and Chief Operating Officer, AstraZeneca KK (Japan), Shanghai, China
|
|
2012 Sixth International Pharma Congress Keynote Address by
|

Christopher A. Viehbacher
Chief Executive Officer, Sanofi, Chairman, Genzyme, Paris, France
|
|
2011 Twelfth Pharmaceutical Regulatory Compliance Congress and Best Practices Forum by
|
John C. Lechleiter, PhD
Chairman, President and Chief Executive Officer, Eli Lilly and Company, Chairman-elect, PhRMA, Indianapolis, IN
|
|
2011 Fifth International Pharma Congress Keynote Address by
|

David Brennan
Former Chief Executive Officer, AstraZeneca, President, IFPMA, Past Chairman, PhRMA, London, UK
|
|
FOLLOW INTERNATIONAL PHARMA CONGRESS ON
|


|
|
|
OTHER INTERNATIONAL PHARMA CONGRESSES
|

2007 - Brussels

2008 - Paris

2009 - Rome

2010 - Berlin |

2011 - Istanbul

2011 - Singapore

2012 - Budapest

2012 - Shanghai |

2012 - São Paulo

2013 - Madrid, Spain

2013 - Kuala Lumpur

2014 - Dubai
|
|
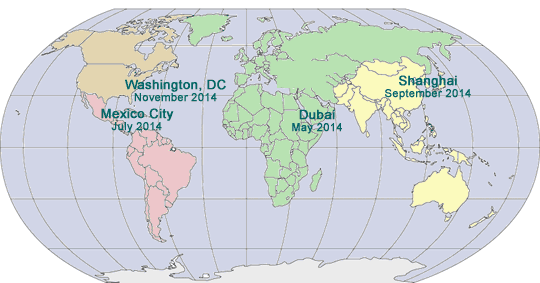
SAVE THE DATES
|
|
SIXTH ANNUAL SUMMIT ON DISCLOSURE, TRANSPARENCY AND AGGREGATE SPEND FOR DRUG, DEVICE AND BIOTECH COMPANIES
|
A Hybrid Conference and Internet Event
Sponsored by Pharmaceutical Compliance Forum
February 5 - 7, 2014
Hyatt Regency Crystal City
Washington, DC
www.DisclosureSummit.com
|
|
SECOND LATIN AMERICAN PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
|
A Hybrid Conference and Internet Event
Sponsored by Latin American Ethics and Compliance Network
Cosponsored by International Society of Healthcare Compliance Professionals (ETHICS) and Pharmaceutical Compliance Forum (PCF)
July 28 - 31, 2014
Mexico City, Mexico
www.LatinAmericanPharmaCongress.com
|
|
FOURTH ASIA PACIFIC PHARMACEUTICAL
COMPLIANCE CONGRESS
|
A Hybrid Conference and Internet Event
Sponsored by Asia Pacific Healthcare Industry Compliance Team
Cosponsored by International Society of Healthcare Ethics and Compliance Professionals (ETHICS) and Pharmaceutical Compliance Forum (PCF)
September, 2014
Beijing, China
www.AsianPharmaCongress.com
|
|
FIFTEENTH PHARMACEUTICAL REGULATORY
AND COMPLIANCE CONGRESS
|
A Hybrid Conference and Internet Event
Transformational Learning - Effective Knowledge Exchange
Sponsored by Pharmaceutical Compliance Forum
November 3 - 5, 2014
Hyatt Regency on Capitol Hill
Washington, DC
www.PharmaCongress.com
|
|
|
NEW PARTICIPATION OPTION: LIVE AND ARCHIVED INTERNET ATTENDANCE
|
|
Announcing a new way to participate in the International Pharma Congress: You can now register to watch the Congress in live streaming video over the Internet and at your convenience at any time 24/7 for the six months following the event.
|
|
|
TRADITIONAL ONSITE ATTENDANCE
|
|
Simply register, travel to the conference city and attend in person.
Pros: subject matter immersion; professional networking opportunities; faculty interaction
|
|
|
|
LIVE AND ARCHIVED WEBCAST ATTENDANCE
|
|
Watch the conference in live streaming video over the Internet and at your convenience
at any time 24/7 for the six months following the event.
The archived conference includes speaker videos and coordinated PowerPoint presentations.
Pros: Live digital feed and 24/7 Internet access for next six months; Accessible in office, at home or anywhere worldwide with Internet access; Avoid travel expense and hassle; No time away from the office
|
|
|
WEBCAST INTERFACE SAMPLE
|
Click here for a sample stream
|
|
|
|
|
|
|
|