|
|
|
Press Release: Ninth International Pharma Compliance Congress Features a Global Transparency Panel
|

- A Hybrid Conference and Internet Event
- Sponsored by ethics, the International Society of Healthcare Compliance Professionals
- Co-sponsored by the Pharmaceutical Compliance Forum
- May 11 - 13, 2015
- Onsite at the Crowne Plaza Brussels - Le Palace in Brussels, Belgium
- In Your Own Office or Home live via the Internet with 24/7 Access for Six Months
- www.InternationalPharmaCongress.com
PRESS RELEASE
Phone: 800-503-8171
Email: registration@hcconferences.com
Website: www.InternationalPharmaCongress.com
|
|
FEATURING A GLOBAL TRANSPARENCY PANEL BY
|

Holger Diener, PhD
Managing Director, Association of Voluntary, Self-Regulation for the Pharmaceutical Industry, Berlin, Germany |

George Fife
Executive Director, Compliance & Ethics, Bristol-Myers Squibb, Former, Compliance & Internal Audit Leader, EMEA, GE Healthcare, Paris, France |

Dr. Gudula Petersen
Chief Compliance Officer Europe & Australia, Grunenthal, Aachen, Germany
|

Jose F. Zamarriego Izquierdo
Director Unidad de Supervision Deontologica, FARMAINDUSTRIA, Madrid, Spain |

Dr. Michael Bartke
Director Compliance Management, DAIICHI SANKYO EUROPE GmbH, Co-chair, EFPIA Compliance Workgroup, Munich, Germany, (Moderator)
|
|
|
|
WASHINGTON DC USA -- CME UPDATE NEWS SERVICE™ -- JANUARY 13, 2015: The Ninth International Pharmaceutical Compliance Congress, www.InternationalPharmaCongress.com, will take place on May 11 - 13, 2015 at the Crowne Plaza Brussels - Le Palace in Brussels, Belgium. Registrants may attend the event in person or online (live webcast and archived for 6 months).

|
KEYNOTE SPEAKERS
|

Robert Barrington, PhD
Executive Director, Transparency International-UK, London, UK |

Richard Bergström
Director General, European Federation of Pharmaceutical Industries and Associations (EFPIA), Former Director General, LIF Sweden, Brussels, Belgium
|
|
|
|
FEATURING AN EFPIA STAKEHOLDER WORKSHOP
|
 The European Federation of Pharmaceutical Industries and Associations (EFPIA) is offering an independent EFPIA STAKEHOLDER WORKSHOP on Monday, May 11, 2014.
The European Federation of Pharmaceutical Industries and Associations (EFPIA) is offering an independent EFPIA STAKEHOLDER WORKSHOP on Monday, May 11, 2014.
|
|
FEATURED FACULTY
|

Ted Acosta, Esq.
Principal, Fraud Investigation & Dispute Services, EY, Former Senior Counsel, Office of Inspector General, United States Department of Health and Human Services, New York, NY, USA and Paris, France |

Andy Bender
President and Founder, Polaris, New York, NY, USA |

Flavia Bollinger
Head Integrity and Compliance MENA (Middle East, North Africa), Novartis Pharma Services AG, Dubai, UAE
|

Fiona Carlin, LLB
Managing Partner, European & Competition Law Practice, Baker & McKenzie, Brussels, Belgium |

Holger Diener, PhD
Managing Director, Association of Voluntary, Self-Regulation for the Pharmaceutical Industry, Berlin, Germany |

Kip Ebel, MBA
Principal, Ernst & Young, New York, NY
|

Sue Egan
Director and Principal Consultant, Sue Egan Associates, Former Vice President Compliance, AstraZeneca, Great Missenden, Buckinghamshire, UK |

George Fife
Executive Director, Compliance & Ethics, Bristol-Myers Squibb, Former, Compliance & Internal Audit Leader, EMEA, GE Healthcare, Paris, France |

Martin Fürle
Vice President, General Counsel & Chief Compliance Officer, DAIICHI SANKYO EUROPE GmbH, Munich, Germany
|

Gary F. Giampetruzzi, Esq.
Partner, Paul Hastings, Former Vice President and Assistant General Counsel, Head of Government Investigations, Pfizer Inc., New York, NY |

Ulf H. Grundmann, JD
Partner, Life Science Practice Group, King and Spalding, Frankfurt, Germany |

Thomas K. Hauser, LLM
Chief Compliance Officer, Siemens Healthcare, Nürnberg, Germany
|

Erinn Hutchinson
Partner, Advisory Services, PricewaterhouseCoopers, Philadelphia, PA |

Isaure Kergall-Gayet, DEA-Law
Compliance & Business Integrity Director France, Sanofi, Former VP, Chief Ethics & Compliance Officer, Ipsen, Former, Director, Ethics & Compliance Officer, Lilly France, Paris, France |

Keith M. Korenchuk, JD, MPH
Partner, Arnold & Porter LLP, Washington, DC
|

Daniel A. Kracov, Esq.
Partner and Head, FDA and Healthcare Practice, Arnold & Porter, Washington, DC, USA |

Aline Lautenberg
General Counsel - Director Legal & Compliance, Eucomed, EDMA and MedTech Europe, Brussels, Belgium |

Paul J. Melling, Esq.
Founding Partner, Baker & McKenzie - CIS, Limited, Moscow, Russia
|

John Patrick Oroho, Esq.
Executive Vice President and Chief Strategy Officer, Porzio Life Sciences, LLC, Principal, Porzio, Bromberg & Newman PC, Morristown, NJ, USA |

Dr. Gudula Petersen
Chief Compliance Officer Europe & Australia, Grunenthal, Aachen, Germany |

Brian Riewerts
Partner, Global Pharmaceuticals and Life Sciences, PwC LLP, Baltimore, MD, USA
|

Christine Sainvil
Compliance Officer, EthicalMedTech, Brussels, Belgium |

Brian Sharkey, Esq.
Counsel, Porzio, Bromberg & Newman, Director of Compliance and Legal Affairs, Porzio Governmental Affairs, LLC, Morristown, NJ, USA |

Heather Simmonds
Director, Prescription Medicines Code of Practice Authority; Vice-Chair, IFPMA Code Compliance Network, London, UK
|

Melda Tanyeri, MS, MBA
Director, Fraud Investigation & Dispute Services, Ernst & Young, London, UK |

Joseph B. Tompkins, Jr.
Partner, Sidley Austin LLP, Former Deputy Chief of the Fraud Section, Criminal Division, United States Department of Justice, Washington, DC, USA |

Dumitru Uta, MD, MBA, CCEP
Ethics and Compliance Director, South-East Europe, Eli Lilly and Company, Bucharest, Romania
|

Frank Wartenberg, PhD
President, Central Europe, IMS Health, Frankfurt/Main, Germany |

Mariusz Witalis
Partner, Fraud Investigation and Dispute Services, Ernst & Young LLP, Warsaw, Poland
|

Elisabethann Wright, Esq.
Partner, Hogan Lovells, Former Senior Legal Officer and Hearing Officer, EFTA Surveillance Authority, Brussels, Belgium |

Jose F. Zamarriego Izquierdo
Director Unidad de Supervision Deontologica, FARMAINDUSTRIA, Madrid, Spain
|
|
|
|
OTHER INTERNATIONAL PHARMA CONGRESSES
|

2007 - Brussels

2008 - Paris

2009 - Rome

2010 - Berlin |

2011 - Istanbul

2011 - Singapore

2012 - Budapest

2012 - Shanghai |

2012 - São Paulo

2013 - Madrid

2013 - Kuala Lumpur

2014 - Dubai
|

2014 - Mexico City
|
|
|
|
|
|
SPEAKER PRESENTATION PROPOSALS
|
Speaker/Presentation Proposals for the International Pharma Congress May Be Submitted Through Our Online Form
- Click Here -
|
|
|
|
SPONSORED BY:
|
|

|
|
CO-SPONSORED BY:
|
|

|
|
CONGRESS CO CHAIRS
|

Ann Beasley
Chief Compliance Officer, Biogen Idec International, GmbH, Former Global Compliance Officer, Novartis Pharma AG, Board Member and Co-chair, Strategic Committee, International Society of Healthcare, Ethics and Compliance Professionals (ETHICS), Cambridge, MA, USA
|

Dr. Michael Bartke
Director Compliance Management, DAIICHI SANKYO EUROPE GmbH, Co-chair, EFPIA Compliance Workgroup, Munich, Germany
|

Dominique Laymand, Esq.
President, International Society of Healthcare Ethics, and Compliance Professionals (ETHICS), Executive Director Compliance & Ethics EMEA, (Europe, Middle-East, Africa, Russia and Turkey), Bristol-Myers Squibb, Paris, France
|

Roeland Van Aelst
Regional Vice President - HCCO MD&D EMEA & Canada, Office Health Care Compliance and Privacy, Johnson & Johnson, Board Member, International Society of Healthcare, Ethics and Compliance Professionals (ETHICS), Chairman, MedTech Compliance Network, Brussels, Belgium
|
|
|
|
GRANTORS:
|
|
BRONZE
|





|
|
FOLLOW INTERNATIONAL PHARMA CONGRESS ON
|


|
|
INTERNATIONAL PHARMA CONGRESS IS
|

|
|
PARTICIPATION OPTIONS
|
|
|
|
TRADITIONAL ONSITE ATTENDANCE
|
Simply register, travel to the conference city and attend in person.
Pros: subject matter immersion; professional networking opportunities; faculty interaction

|
|
LIVE AND ARCHIVED WEBCAST PARTICIPATION
|
Watch the conference in live streaming video over the Internet and at your convenience at any time 24/7 for the six months following the event.
The archived conference includes speaker videos and coordinated PowerPoint presentations.
Pros: Live digital feed and 24/7 Internet access for next six months; Accessible in office, at home or anywhere worldwide with Internet access; Avoid travel expense and hassle; No time away from the office


|
WEBCAST INTERFACE SAMPLE
|
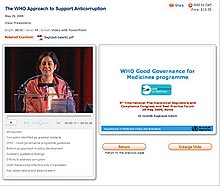
Click here for a sample stream
|
|

 This site complies with the HONcode standard for trustworthy health information: This site complies with the HONcode standard for trustworthy health information:
verify here.


|
|
|
|
|
|