|
|
|
PCF Virtual 21st Anniversary Pharmaceutical and Medical Device Compliance Congress Features DOJ Keynote, FCPA Update and AUSA Roundtable
|
- Virtual Online Video Event Live and 6 Months Access to Video Archive
- Sponsored by the Pharmaceutical Compliance Forum
- Media Partners: Harvard Health Policy Review, Health Affairs and Life Sciences Compliance Update
- November 4 - 6, 2020
- www.PharmaCongress.com
PRESS RELEASE
Phone: 800-503-7419
Email: reginfo@hcconferences.com
Website: www.PharmaCongress.com
|
|
AGENDA NOW AVAILABLE
|
|
Click here to view the agenda.
|
|
SPECIAL PCF REGISTRATION DISCOUNTS
|
|
Click here for PCF member reg discounts.
|
|
|
|
FEATURING US DOJ KEYNOTE ADDRESS
|

Gustav W. Eyler, JD
Director, Consumer Protection Branch, Civil Division, US Department of Justice, Washington, DC |

Andy Mao, JD
Deputy Director, Fraud Section, Civil Division, Elder Justice Initiative Coordinator, US Department of Justice, Washington, DC |

Gejaa T. Gobena, JD
Partner, Hogan Lovells; Former Deputy Chief, Fraud Section, Criminal Division; Former Trial Attorney, Civil Division, Fraud Section, US Department of Justice; Washington, DC
|
|
|
US DOJ AND US SEC UPDATE ON FCPA ENFORCEMENT
|

Craig Carpenito, JD
United States Attorney, US Attorney's Office, District of New Jersey; Former Senior Counsel, Northeast Regional Office, US Securities and Exchange Commission; Newark, NJ |

Robert I. Dodge, JD
Assistant Director, FCPA Unit, US Securities & Exchange Commission; Former Assistant U.S. Attorney, US Attorney's Office, Western District of Michigan; Former Assistant Section Chief, Environmental Defense Section, US Department of Justice; Washington, DC |

David Last, JD
Assistant Chief, FCPA Unit, Fraud Section, Criminal Division, US Department of Justice, Washington, DC |

Gary F. Giampetruzzi, Esq.
Partner, Paul Hastings; Former Vice President and Assistant General Counsel, Head of Government Investigations, Pfizer Inc.; New York, NY
|
|
|
THE ANNUAL AUSA ROUNDTABLE
|

Matthew J. Lash, JD
Assistant Director, Consumer Protection Branch, US Department of Justice, Washington, DC |

Gregg Shapiro, JD
Assistant US Attorney and Chief, Affirmative Civil Enforcement Unit, US Attorney's Office, District of Massachusetts, US Department of Justice, Boston, MA |

Amanda Masselam Strachan, JD
Assistant US Attorney and Chief, Health Care Fraud Unit, US Attorney's Office, District of Massachusetts, US Department of Justice, Boston, MA |

John T. Bentivoglio, JD
Partner, Skadden Arps LLP; Former Special Counsel for Healthcare Fraud, and Chief Privacy Officer, US Department of Justice; Washington, DC
|
|
AND FEATURING THE PHARMA CONGRESS ADVANCED VIDEO BROADCAST PLATFORM
|
|
|
|
CO CHAIRS
|

Indrani Franchini, JD
Executive Vice President Chief Compliance Officer, Alexion Pharmaceuticals, New York, NY (PCF CO-Chair) |

Jeffrey M. Kawalek, MBA
Deputy Chief Compliance Officer, Jazz Pharmaceuticals, Inc., Chair, Pharmaceutical Compliance Forum (PCF), Philadelphia, PA (PCF Chair) |

Margaret Sparks, JD
Associate Vice President, North America Ethics and Business Integrity, Sanofi US, Bridgewater, NJ (PCF Co-Chair)
|

Ann Marie Tejcek
Senior Director, Chief Compliance Officer North America, Eli Lilly, Indianapolis, IN (PCF Co-Chair) |

Donna White, CCEP
Vice President, Compliance, Chiesi, Cary, NC (PCF Co-Chair) |

Joe Zimmerman
Vice President, Chief Compliance Officer & Privacy Officer, Ferring Pharmaceuticals, Inc., Parsippany, NJ (PCF Co-Chair)
|
|
UPDATED AGENDA AT A GLANCE
(sessions subject to change)
|
|
|
CONCURRENT CCO ROUNDTABLE AND MINI SUMMITS I & II
INVITATION-ONLY, CLOSED CCO ROUNTABLE (10 am EST - 12:15 pm EST)
|
|
10:00 am |
Welcome & Introductions
|
|
10:10 am |
Leveraging AI, Predictive Analytics and Other Technologies to Conduct Oversight and Enforcement
|
|
10:40 am |
PhRMA and AdvaMed Updates
|
|
11:00 am |
CCO Connection Program-Partner Experience
|
|
11:05 am |
Interview with the Department of Justice: Safeguarding the High-Risk Activity of Speaker Programs
|
|
11:35 am |
Facilitated, Interactive Discussion Video Roundtables on Promotional Programs
|
|
12:00 pm |
Sharing from Roundtable Discussions
|
|
12:15 pm |
Adjournment
|
|
MINI SUMMITS I & II
|
|
MINI SUMMITS I (10 am EST - 11 am EST)
|
|
|
- I: State-of-the-Art Compliance Training (in Dynamic Times)
- II: Key Learnings in Electronic Health Record Enforcement Actions
- III: Key Considerations and Best Practices in Operationalizing Speaker Programs
- IV: Privacy, GDPR and California Consumer Privacy Act – What’s Next
|
|
MINI SUMMITS II (11 am EST - 12:15 pm EST)
|
|
|
- V: Third Party Management Beyond Due Diligence: Selecting, Integrating and Managing Third Parties throughout the Relationship Lifecycle
- VI: FDA Regulatory Updates for Compliance Professionals
- VII: Best Practices in Operationalizing Updates to the Sunshine Law
- VIII: Compliance Considerations for Rare and Ultra Rare Drugs
|
|
OPTIONAL LIVE INTERACTIVE TOPIC DISCUSSIONS
(12:15 pm EST - 1:00 pm EST)
(Open to all; May move among sessions.)
|
|
OPENING PLENARY SESSION (1 pm EST - 5 pm EST)
|
|
1:00 pm |
Welcome and Introduction: PCF Co-Chairs
|
|
1:15 pm |
Keynote: OIG Update
|
|
2:00 pm |
US DOJ Keynote
|
|
3:15 pm |
FDA Keynote
|
|
3:45 pm |
Annual Chief Compliance Officer Fireside Chat
|
|
5:00 pm |
Adjournment
|
|
THURSDAY, NOVEMBER 5, 2020
|
|
CONCURRENT MINI SUMMITS III & IV
MINI SUMMITS III (10 am EST - 11 am EST)
|
|
|
- IX: Managing Monitorships and Independent Review Organizations to Maximize Effectiveness
- X: Building Ethics and Integrity into the Foundation of Your Compliance Program
- XI: Annual Medical Device Roundtable
- XII: Update on Pharma CIAs, including Changes to OIG’s Model CIA Template and Insights on Agency Priorities from Recent CIA Negotiations
- XIII: Drug Promotion from the Living Room: Navigating Interactions with HCPs and Addressing Compliance Concerns during a Pandemic
- XIV: Changing Dynamics of State Price Transparency and Reporting Requirements
|
|
MINI SUMMITS IV (11:15 am EST - 12:15 pm EST)
|
|
|
- XV: Compliance Challenges and Opportunities for Emerging Organizations
- XVI: Keeping a Pulse on Social Media Communication, Including Promotion of Products Across Borders
- XVII: Compliance Risk Assessment Key Insights and Alignment to Enterprise Risk Management
- XVIII: Government Enforcement Trends
- XIX: Best Practices of Conducting Investigations
- XX: Patient Services Compliance Survey (Year 4) Update
|
|
OPTIONAL LIVE INTERACTIVE TOPIC DISCUSSIONS (12:15 pm EST - 1:00 pm EST)
(Open to all; May move among sessions.)
|
|
PLENARY SESSION (1 pm EST - 5 pm EST)
|
|
1:00 pm |
Welcome and Introduction: PCF Co-Chairs
|
|
1:15 pm |
Equity, Diversity and Inclusion
|
|
1:45 pm |
Implementing, Equity, Diversity, and Inclusion Roundtable
|
|
2:30 pm |
Visit Exhibit Hall
|
|
3:00 pm |
US DOJ and US SEC Update on FCPA Enforcement
|
|
3:45 pm |
Advanced Patient Engagement Strategies
|
|
4:30 pm |
Adjournment
|
|
CONCURRENT MINI SUMMITS V & VI
|
|
MINI SUMMITS V (10 am EST - 11 am EST)
|
|
|
- XXI: Meaningful Board Engagement and Support for Board Oversight of the Compliance Program
- XXII: How would you Respond to the DOJ’s Fundamental Question, “Does the Corporation’s Compliance Program Work” in Practice?
- XXIII: Best Practices from Enforcement Actions on Fraud and Misuse of Patient Support Programs — XXIV: DOJ’s Fundamental Question: “Does the Corporation’s Compliance Program Work” in Practice?
- XXIV: SEC Compliance: Living in the Material (Non-Public Information) World
- XXV: Reflections on a Successful Co-Promotion Partnership
|
|
MINI SUMMITS VI (11:15 am EST - 12:15 pm EST)
|
|
|
- XXVI: Compliance Challenges with Digital Health Applications
- XXVII: Managing Communication Issues and Leadership during a Crisis or an Emerging Post-Crisis Environment
- XXVIII: Fair Market Value Calculations, Benchmarks and New Standards in a Virtual World
- XXIX: Compliance Monitoring in a Virtual World
- XXX: Fraud and Abuse Risks Related to COVID-19 Relief
- XXXI: Research and Development Compliance Challenges
|
|
OPTIONAL LIVE INTERACTIVE TOPIC DISCUSSIONS (12:15 pm EST - 1:00 pm EST)
(Open to all; May move among sessions.)
|
|
|
CLOSING PLENARY SESSION (1 pm EST - 4 pm EST)
|
|
1:00 pm |
Welcome and Introduction: PCF Co-Chairs
|
|
1:15 pm |
Roundtable on Implications of the Election for Lifesciences, including Pricing and Cost Containment Policy
|
|
2:00 pm |
Using Data Analytics and Automation to Drive Smarter, More Effective and Cost-Effective Compliance Programs
|
|
2:45 pm |
Annual AUSA Roundtable
|
|
3:00 pm |
The Post-Pandemic Compliance Department of the Future: Virtual, Ethics, Integrity and More
|
|
3:45 pm |
Adjournment
|
|
|
WASHINGTON, DC USA -- PHARMA UPDATE NEWS SERVICE™ -- OCTOBER 21, 2020: The 21st Anniversary Pharmaceutical and Medical Device Compliance Congress, www.PharmaCongress.com, to be held November 4 - 6, 2020 as a fully virtual event, today announced the conference dates and a call for presentation proposals.
|
|
VIRTUAL CONGRESS HIGHLIGHTS
|
- New content, optimized for online learning
- Focused on new regulatory developments
- Managing ethics and compliance programs during the COVID 19 pandemic
- Engage through polling, Q&A sessions, chat and direct video messaging
- Network in real time with other attendees through direct attendee to attendee and group video messaging
- Live and curated sessions streamed directly to you through our easy to navigate virtual platform
- Access to an extensive video archive of past Congress presentations
- Virtual exhibit hall featuring innovative solutions, products and services
|
|
KEYNOTE SPEAKERS
|

Craig Carpenito, JD
United States Attorney, US Attorney’s Office, District of New Jersey; Former Senior Counsel, Northeast Regional Office, US Securities and Exchange Commission; Newark, NJ |

Susan Dentzer
Visiting Fellow, Duke-Margolis Center for Health Policy; Former Editor in Chief, Health Affairs; Former Health Correspondent, PBS NewsHour; Washington, DC |

Robert I. Dodge, JD
Assistant Director, FCPA Unit, US Securities & Exchange Commission; Former Assistant U.S. Attorney, US Attorney’s Office, Western District of Michigan; Former Assistant Section Chief, Environmental Defense Section, US Department of Justice; Washington, DC
|

Robert W. Dubois, MD, PhD
Interim President and CEO, National Pharmaceutical Council (NPC); Former Chief Medical Officer, Cerner LifeSciences; Washington, DC |

Gustav W. Eyler, JD
Director, Consumer Protection Branch, Civil Division, US Department of Justice, Washington, DC |

Catherine (Katie) Gray, PharmD
Acting Director, Office of Prescription Drug Promotion (OPDP), US Food and Drug Administration, Silver Spring, MD
|

Matthew J. Lash, JD
Trial Attorney, Consumer Protection Branch, US Department of Justice, Washington, DC |

David Last, JD
Assistant Chief, FCPA Unit, Fraud Section, Criminal Division, US Department of Justice, Washington, DC |

Andy Mao, JD
Deputy Director, Fraud Section, Civil Division, Elder Justice Initiative Coordinator, US Department of Justice, Washington, DC
|

Dan Mendelson, MPP
Founder, Avalere Health, Operating Partner, Welsh, Carson, Anderson & Stowe, Board, Centrexion Therapeutics Corp., Washington, DC |

Mary E. Riordan, JD
Senior Counsel, Office of Counsel to the Inspector General, Office of Inspector General, US Department of Health and Human Services, Washington, DC |

Gregg Shapiro, JD
Assistant US Attorney and Chief, Affirmative Civil Enforcement Unit, US Attorney's Office, District of Massachusetts, US Department of Justice, Boston, MA
|

Amanda Masselam Strachan, JD
Assistant US Attorney and Chief, Health Care Fraud Unit, US Attorney’s Office, District of Massachusetts, US Department of Justice, Boston, MA |

Edie Stringfellow, MS
Director, Diversity & Inclusion, Massachusetts Biotechnology Council, Cambridge, MA
|
|
|
FEATURING THE ANNUAL INVITATION-ONLY CHIEF COMPLAINCE OFFICER ROUNDTABLE
|
|
November 4, 2020; Invitation-only; No Cost. CCOs interested in attending may contact Debra Scanlon, Administrator, Pharmaceutical Compliance Forum at info@pharmacomplianceforum.org.
|
|
|
AdvaMed UPDATE
|

Christopher L. White, JD
Chief Operating Officer & General Counsel, Advanced Medical Technology Association (AdvaMed), AdvaMed COVID Action Team Leader, Washington, DC
|
|
|
|
PhRMA UPDATE
|

Julie Ritchie Wagner, JD
Assistant General Counsel, PhRMA; Former Senior Counsel, Office of Counsel to the Inspector General, US Department of Health and Human Services (HHS); Washington, DC
|
|
|
|
OIG ANALYTICS/AI UPDATE
|

Renata Maziarz Miskell, MPS
Acting Chief Data and Analytics Officer, Office of Inspector General (OIG), US Department of Health and Human Services, Washington, DC
|
|
|
|
DOJ SPEAKER PROGRAMS UPDATE
|

Jake Lillywhite, JD
Assistant US Attorney, Civil Division, US Attorney's Office, Southern District of New York, US Department of Justice, New York, NY
|
|
|
|
FEATURING THE ANNUAL CHIEF COMPLIANCE OFFICER FIRESIDE CHAT WITH
|

Dennis K. Barnes, JD CPA
Vice President, Global Governance, Risk & Compliance, Mayne Pharma; Former Vice President, Compliance & Risk Management, Global Compliance Officer, PAREXEL International; Raleigh-Durham, NC |

Mark Graves, JD, MBA
Senior Vice President and Chief Compliance Officer, MiMedx; Former Director, Global Patient Experience & Value, UCB; Former Senior Director, Pharmaceutical Products Division, Abbott; Marietta, GA |

Nancy A. Grygiel, JD
Senior Vice President, Chief Compliance Officer, Amgen; Former VP Compliance, Corporate & International, Allergan; Former Senior Director Global Compliance, Mylan; Newbury Park, CA
|

John Knighton, JD
Chief Compliance Officer, TherapeuticsMD; Former Vice President, Head of Global Compliance, Orexigen Therapeutics; Boca Raton, FL |

Tara R. Shewchuk, JD, LLM
Vice President and Deputy, Office of Ethics & Compliance, Medtronic; Former Senior Director, Ethics and Compliance, Abbott (now AbbVie); Former VP, Chief Compliance, Privacy & Security Officer, Resurrection Health Care (now Ascension/AMITA); Minneapolis, MN |

Tom Gregory, MBA
Partner, Forensic & Integrity Services, EY, Atlanta, USA (Moderator)
|
|
|
AND A ROUNDTABLE ON IMPLEMENTING EQUITY, DIVERSITY AND INCLUSION BY
|

Michael R. Clarke, CCEP
Vice President, Global Chief Compliance Officer, ConvaTec; Former VP, Corporate Compliance, Indivior; Former VP, Ethics & Compliance, Americas, Actavis; Former VP & Compliance Officer, Biomet Spine & Bone Healing Technologies; Bridgewater, NJ |
Sujata Dayal, JD
Vice President & Global Chief Compliance Officer, Medline Industries; Former VP Healthcare Compliance & Privacy, Johnson & Johnson; Former Global Chief Compliance Officer and Corporate VP, Biomet; Chicago, IL |

Jill Fallows Macaluso
Corporate Vice President, Chief Ethics, Compliance & Privacy Officer, NovoNordisk, Princeton, NJ
|

Robert A. Ladd, JD
Vice President, Global Head Integrity & Compliance, Novartis, Oncology; Former General Counsel, GE Healthcare; Former Global Head of Compliance Training, Goldman Sachs; Former Senior Corporate Counsel, Pfizer; New York, NY |

Latarsha Stewart, JD, MS
Vice President, Head of Compliance, Servier Pharmaceuticals; Former Head of Compliance, Global Oncology Business Unit, Takeda Oncology; Boston, MA
|
|
|
|
|
|
|
FEATURED FACULTY
|

Sergio Juan Abreu
Regional Compliance Officer, APAC, Merck Group; Former Regional Compliance Officer – LATAM, Merck Serono; Singapore |

Peter Agnoletto, CPA
Compliance Officer, Primary Care and Consumer Health Care, Sanofi; Former Chief Compliance Officer & Chief Audit, Executive & ERM Leader, Par Pharma; Flemington, NJ |

Khelin N. Aiken, JD
Partner, Baker & McKenzie LLP; Former Regulatory Counsel, Center for Drug Evaluation and Research, US Food and Drug Administration (FDA), Washington, DC |

Sergio Alegre, JD
Vice President, Global Compliance, Osmotica; Former Executive Director Compliance, Pacira; Bridgewater, NJ |

Punkaj T. Amin, MBA, CFE
Compliance Officer - US Wound Management,Office of Ethics and Compliance (OEC), Smith & Nephew; Former Director, Communications & Public Relations - North America, Grifols; Fort Worth, TX
|

Melia Arnett-Archie, JD
Senior Privacy & Data Protection Counsel, Stryker; Former Assistant State Attorney, Miami-Dade State Attorney’s Office; Fort Lauderdale, FL |

Samantha Barrett Badlam, JD
Counsel, Litigation and Enforcement Group, Ropes & Gray, Washington, DC |

Ann E. Beasley, JD
Director, Life Sciences, Governance, Risk & Compliance, Navigant; Former Senior Vice President, Chief Compliance Officer, Biogen; Boston, MA |

John T. Bentivoglio, JD
Partner, Skadden Arps LLP; Former Special Counsel for Healthcare Fraud, and Chief Privacy Officer, US Department of Justice; Washington, DC |

Richard Bistrong, MA
Chief Executive Officer, Front-Line Anti-Bribery LLC, Contributing Editor, FCPA Blog; Former Confidential Human Source (CHS) and Cooperating Witness, FBI and US DOJ; Former Cooperating Witness, City of London Police, HMRC and CPS, UK; New York, NY
|

Craig Bleifer, JD
Principal, Craig B. Bleifer, LLC; Former Corporate Vice President, General Counsel North America, Novo Nordisk; Former Senior Vice President, General Counsel and Secretary, Daiichi Sankyo; Summit, NJ |

Joseph Calarco
Vice President, Compliance, Nabriva Therapeutics; Former Chief Compliance Officer, NEOS Therapeutics; Former Director, Compliance & Ethics, US Operations, Bristol-Myers Squibb; Lansdale, PA |

Christie Camelio
Chief Compliance, Ethics & Risk Management Officer, TG Therapeutics; Former Vice President, Deputy Global Chief Compliance Officer, Celgene; New York, NY |

Cynthia Cetani
Chief Integrity & Compliance Officer, Indivior; Former Group Integrity & Compliance, Head, Compliance Operations, Novartis International AG; Richmond, VA |

Parth Chanda, JD, MPA
Founder and CEO, Lextegrity; Former Chief Compliance Counsel, Oncology, Pfizer; New York, NY
|

Katherine Chaurette, MS, JD
Vice President Healthcare Law and Compliance, Blueprint Medicines; Former Sr. Director, Regulatory and Commercial Law, Regeneron; Former VP, Legal, Head of Health Law, Regulatory, Market Access, Marketed and Launch Products, Sanofi; Jamaica Plain, MA |

Masha Chestukhin, MSJ
Associate Director, Compliance Officer R&D, IA, FMV, Sanofi Genzyme; Former Senior Manager NA Compliance, Sanofi; Jamaica Plain, MA |

Yvonne Clark, JD
Corporate Compliance Counsel, Spark Therapeutics; Former Commercial Counsel, Merck; Philadelphia, PA |

Thomas Costa, JD
Member, US Board of Directors, Sanofi; Former Vice President, US Compliance & Ethics, Bristol-Myers Squibb; Washington, DC |

Clarissa Crain
Senior Manager, Deloitte; Former Analyst, Commercial Operations, Cephalon; Philadelphia, PA
|

Emily Cunningham
Associate Principal, Compliance Consulting, IQVIA, New York, NY |

Meenakshi Datta, JD
Partner and Global Co-leader, Healthcare Practice, Sidley Austin LLP, Chicago, IL |

BJ D'Avella, MBA
Group Leader, Life Sciences Consulting Group, Paul Hastings, New York, NY |

Anisa Dhalla
Global Chief Compliance Officer, UCB, Inc.; Former Manager, Regulatory Affairs, Solvay and Mikart; Atlanta, GA |

Sarah diFrancesca, JD
Partner, Dinsmore & Shohl LLP, Cincinnati, OH
|

Stefanie A. Doebler, JD
Of Counsel and Co-chair, Health Care Practice Group, Covington & Burling LLP, Washington, DC |

Tara D'Orsi, JD
Executive Vice President, Chief Compliance Officer and General Counsel, Kyowa Kirin North America; Former Senior Counsel, Reliant Pharmaceuticals; New York, NY |

J. Mark Farrar, CPA, CFE, CFF
Managing Director, Navigant; Former Interim Global Chief Compliance Officer, Beckman Coulter; Atlanta, GA |

Margaret Feltz, MA, JD
Vice President, Ethics & Compliance, Purdue Pharma; Former Compliance Counsel, Boehringer Ingelheim; Stamford, CT |

Christine Fiore, MBA
Executive Director, Ethics and Commercial Compliance, Boehringer Ingelheim; Former Group Director Ethics and Compliance, Smith & Nephew; Ridgefield, CT
|

Hollie K. Foust, JD
Senior Vice President, Legal and Compliance, Cardinal Health,Dublin, OH |

Nereyda Garcia, JD
Compliance Head, Rare Disease and Rare Blood Disorder, Sanofi; Former Global Head, Ethics and Compliance, Alnylam; Former Sr. Director, Compliance, Biogen Idec; Boston, MA |

Gary F. Giampetruzzi, Esq.
Partner, Paul Hastings; Former Vice President and Assistant General Counsel, Head of Government Investigations, Pfizer Inc.; New York, NY |

Thomas M. Glavin, JD
Chief Compliance Officer for the Americas, Olympus Corporation of the Americas; Former Vice President, US/Americas Compliance Officer, Shire; Center Valley, PA |

Jonathan Glazier, MBA, JD
Head of Legal Compliance, Philips North America; Former Sr Director of Corporate Compliance and Privacy Officer, Fresenius Medical Care North America; Andover, MA
|

Betania Glorio, LLM
Global Compliance Officer, Healthcare, Merck KGaA; Former Senior Director, Legal and Compliance EMEA, Horizon, Raptor and Onyx; Darmstadt, Germany |

Gejaa T. Gobena, JD
Partner, Hogan Lovells; Former Deputy Chief, Fraud Section, Criminal Division; Former Trial Attorney, Civil Division, Fraud Section, US Department of Justice; Washington, DC |

Thomas Goimarac, MBA, CHFP
Managing Consultant, Berkeley Research Group, LLC (BRG), Phoenix, AZ |

Christine Gordon, JD
Deputy Chief Compliance Officer, Olympus Corporation of the Americas; Former Assistant District Attorney, Philadelphia District Attorney’s Office; Bethlehem, PA |

Adam Greene, JD, MPH
Partner and Co-chair, Health Information & HIPAA Practice, Davis Wright Tremaine LLP; Former Senior Health Information Technology and Privacy Specialist, Office for Civil Rights, HHS; Washington, DC
|

Amy J. Greer, JD
Partner, Baker & McKenzie LLP; Former Regional Trial Counsel (Philadelphia), US Securities and Exchange Commission; New York, NY |

Ethan Gumpert
Global Ethics, Compliance and Governance, Advanced Medical Technology Association (AdvaMed), Washington, DC |

Saul B. Helman, MD, MBA
Partner, Life Sciences Functional Practice Leader, Guidehouse; Former Director, Eli Lilly; Zionsville, IN |

Michael Hercz, JD
Senior Vice President and General Counsel, Sentynl; Former Vice President, Law & Chief Compliance Officer, Victory Pharma; Former Executive Director, Enterprise Risk Management, Amgen; Solana Beach, CA |

Casey J. Horton, CFE
Director, Life Sciences, Governance, Risk & Compliance, Navigant, Chicago, IL
|

Emily Huebener, MA
Senior Manager, Health and Life Sciences Forensic & Integrity Services, EY, Chicago, IL |

Briannon Irwin, JD
Senior Managing Consultant, Berkeley Research Group (BRG); Former Judicial Law Clerk, The Supreme Court of Pennsylvania; Washington, DC |

James Jacobson, JD
Senior Data Privacy Counsel and Chief Privacy Officer, U.S, Merck KGaA, Darmstadt, Germany |

Adam Janoff, JD
Senior Compliance Counsel, EMD Serono; Former, Senior Counsel, GE Healthcare; Wayland, MA |

Michael Joachim, JD
Head of Ethics & Business Integrity, Specialty Care, Sanofi Genzyme, Cambridge, MA
|

Marci Juneau, MBA
Partner, Helio Health Group, Atlanta, GA |

Catherine Healy Kanzler
Executive Director, Global Compliance Operations, Olympus Corporation of the Americas, Center Valley, PA |

Ben Kaye-Smith
Senior Vice President Ethics, Risk & Compliance, AveXis; Former Corporate Health Safety Environment Manager; Former Global Head Integrity & Compliance Risk Review & Prevention, Novartis; Switzerland |

Jonathon L. Kellerman
Executive Vice President, Global Chief Compliance Officer – Advisor, Office of Ethics & Compliance, Allergan Aesthetics, an AbbVie company; Former Executive Vice President, Global Chief Compliance Officer; Allergan, Madison, NJ |

Andrea L. Kocharyan, JD
Vice President, Head of Legal & Compliance, Zealand US; Former VP, Head of Legal Affairs, Sumitomo Dainippon Oncology; Former VP, US General Counsel & Compliance Officer, EVO Biologics; Cambridge, MA
|

Keith Korenchuk, MPH, JD
Vice President and Chief Compliance Officer, DH Diagnostics, a Danaher Company, Chevy Chase, MD |

Kirt Kraeuter, MGA
Senior Director, Corporate Compliance at Moderna; Former European Compliance Director, Otsuka; Former, Executive Director, Worldwide Compliance Committee & Monitoring, Bristol-Myers Squibb; Yardley, PA |

Daryl Kreml, JD
Vice President, Chief Compliance Officer and Investigations Counsel, Sage Therapeutics; Former Head, US Compliance & Global Program Management, Biogen; Former Director & Senior Managing Counsel, Int’l Compliance, Boston Scientific; Cambridge, MA |

David Lazarus, JD
Assistant United States Attorney and Unit Chief, US Attorney's Office, District of Massachusetts, US Department of Justice; Former Assistant District Attorney, Bronx District Attorney's Office; Boston, MA |

Nancy Libin, JD
Partner and Co-Chair, Privacy & Security, and Technology Practice, Davis Wright Tremaine LLP; Former Chief Privacy and Civil Liberties Officer, US Department of Justice; Washington, DC
|

Jodi Lindsey, JD
Vice President, Healthcare Counsel and Head of U.S. Compliance, Alkermes; Former Associate Director, Commercial Compliance, Biogen; West Roxbury, MA |

Kari K. Loeser, JD
Senior Corporate Counsel & U.S. Healthcare Compliance Officer, Relypsa, Inc; Former Investigator, U.S. Department of Health & Human Services; Former, Policy Analyst, American Medical Association; Redwood City, CA |

David J. Ludlow, JD
Partner, Sidley Austin LLP, Washington, DC |

Joshua Marks, JD
Vice President, Chief Ethics & Compliance Officer, Boehringer Ingelheim; Former Executive Division Counsel, Corporate Compliance Officer & Asst. Secretary, Ben Venue Laboratories; Ridgefield, CT |

Jill Mason, JD
Chief Ethics and Compliance Officer, Orthofix; Former Senior Global Compliance Director, St. Jude Medical; Lewisville, TX
|

Jane Mathews, LLM, JD
Vice President and Chief Compliance Officer, Lexicon; Former Assistant General Counsel, Sunovion; Former Senior Counsel-Managing Attorney - Head of Litigation, Hoffmann-La Roche; Ringwood, NJ |

Heather McCollum, JD, MHA, CCEP-I
Senior Director, Compliance, Shionogi, Florham Park, NJ |

Jennifer McGee, JD
Vice President & Chief Compliance Officer, Otsuka America Pharmaceutical, Rockville, MD |

Kathleen McGovern, JD
Global Investigator, Healthcare & Crisis Management Advisor, EY; Former Senior Deputy Chief, Fraud Section, US Department of Justice; Washington, DC |

Jenny McVey, MS, PHD
Compliance Risk Strategy & Management, Novo Nordisk, Compliance Lead, Hands International, Editorial Board Member, Policy & Medicine Compliance Update, Princeton, NJ
|

Lauren Miller, JD
Corporate Counsel, Otsuka Pharmaceutical Company; Former Regulatory Counsel, Office of Prescription Drug Promotion, FDA; Washington, DC |

Vahan Minassian, JD
Director, US Promotional Monitoring Lead, Pfizer, Philadelphia, PA |

Patrick Mooty
Head of Compliance, Sumitomo Dainippon Pharma America, Marlborough, MA |

Craig Moran, JD
Senior Investigations Counsel, Stryker, Mahwah, NJ |

Gregory S. Moss, LLB
Executive Vice President, General Counsel and Corporate Secretary, Chief Compliance Officer, Kadmon Holdings, New York, NY
|

Kristin Mykulak, JD
Assistant General Counsel, Litigation & Compliance, Dr. Reddy's Laboratories, Princeton, NJ |

Clint Narver, JD
Assistant Director of the Consumer Protection Branch, US Department of Justice; Former Associate Chief Counsel, US Food and Drug Administration; Washington, DC |

Katie Norris, MPA
Director, West Coast Leader, Life Sciences Governance, Risk Management and Compliance, Guidehouse; Former Senior Director, Compliance & Integrity Programs, NSF Health Sciences; San Francisco, CA |

Daniel O’Connor
Senior Vice President, PharmaCertify, a Division of NXLevel Solutions, New York, NY |

John Patrick Oroho, JD
Executive Vice President and Chief Strategy Officer, Porzio Life Sciences, LLC, Principal, Porzio, Bromberg & Newman, Morristown, NJ
|

Raj D. Pai, JD
Partner, Sidley Austin LLP; Former Attorney, Office of Chief Counsel, US Food and Drug Administration; Washington, DC |

Amy Pawloski
President, Strategic Versatility; Former Global Compliance Operations Officer, Endo; Phoenixville, PA |

Veleka Peeples-Dyer, JD
Partner and Chair, North America Food and Drug, Administration (FDA) Practice Group, Baker & McKenzie LLP, Washington, DC |

Brad Powers, JD
General Counsel and Chief Compliance Officer, Lumos Pharma; Former General Counsel and Chief Compliance Officer, Office of the CEO, NewLink Genetics; Des Moines, IA |

Ariadna Quesada, MPP, MSJ
Compliance Director International EMEA, Hillrom; Former Ethics and Compliance Officer, The Netherlands, AbbVie; Former Compliance Director International, DaVita Kidney Care; Amsterdam, Netherlands
|

John Seungjoo Rah, JD
Partner, DLA Piper, Washington, DC |

Kristin Rand, JD, MA
Head, Corporate Compliance and Global Risk Officer, Moderna; Former Vice President & Chief Compliance Officer, Seattle Genetics; Boston, MA |

Kelly N. "Nikki" Reeves, MPA, JD
Partner and Co-chair, Life Sciences and Healthcare Industry Group, King & Spalding LLP, Washington, DC |

Chapman Richardson
Global Head, Data Consumerization, Sanofi; Former Executive Director, Global Next, Generation Digital, Novartis; Bridgewater NJ |

Russell Rose
Senior, Regulatory & Compliance Life Sciences Leader, Deloitte, Atlanta, GA
|

Kevin Ryan, MS, JD
Vice President, Chief Compliance Officer, ACADIA; Former Director, Ethics & Compliance, Novo Nordisk; San Diego, CA |

Jennifer Santos, MPH, JD
Head of Global Corporate Compliance Operations, Vertex Pharmaceuticals; Former Associate General Counsel, Tufts Medical Center; Boston, MA |

Jonathan Sayegh, JD
Associate Director, Compliance & Ethics, Sunovion, Marlborough, MA |

James Sheehan, JD
Chief, Charities Bureau at Attorney General of New York; Former Chief Integrity Officer, Executive Deputy Commissioner, City of New York Human Resources Administration; Former NY Medicaid Inspector General; Former Associate United States Attorney, US Attorney’s Office for the Eastern District of Pennsylvania; New York, NY |

Tara R. Shewchuk, JD, LLM
Vice President & Deputy, Office of Ethics & Compliance , Medtronic Americas; Former Senior Director, Ethics and Compliance; AbbVie Minneapolis, MN
|

Eric Siegel, MBA, JD
Vice President, Head of Compliance US, Idorsia Pharmaceuticals US Inc.; Former Chief Compliance Officer, Jazz Pharmaceuticals; Former Senior Investigator, Office of the Inspector General, City of Philadelphia; Philadelphia, PA |

Paul Silver
Principal, Regulatory & Compliance Life Sciences Leader, Deloitte Advisory, Deloitte, Atlanta, GA |

Alexis Stroud, CQA, CCEP
Director, Ethics & Compliance: Auditing, Monitoring, and Risk Assessment, Boehringer Ingelheim, President, Fairfield County, CT Chapter, Healthcare Businesswomen’s Association, Stamford, CT |

Carol B. Stubblefield, JD, LLM
Partner, Baker & McKenzie LLP, New York, NY |

Tiffany Tang
Associate Principal, Global Compliance Consulting, IQVIA; Former Senior Manager, Ethics and Compliance, Novo Nordisk; New York, NY
|

Jack Tanselle, MBA
Managing Director, Deloitte & Touche LLP, Indianapolis, IN |

Melissa Tearney, JD
Partner and Co-chair, Litigation Department, Choate, Hall & Stewart, Boston, MA |

Bryan Timer, MA
Director, Data Analytics & Transparency, Merck and Co, Inc., Kenilworth, NJ |

Natasha Trifun, JD
Executive Director, R&D, Quality, Operations, External Funding and Global Medical Compliance, Alexion; Former Brazil Compliance Officer, Pfizer; Boston, MA |

Elizabeth Weiss, JD
Assistant General Counsel, Pfizer; Former Research Chemist, Ortho Clinical Diagnostics; Peapack, NJ
|

Caroline H. West, JD
Global Chief Compliance Officer, Olympus Corporation; Former Senior Vice President, Chief Compliance and Risk Officer, Shire; Philadelphia, PA |

Seth B. Whitelaw, JD, LLM, SJD
President and Chief Executive Officer, Whitelaw Compliance Group, LLC; Editor, Life Science Compliance Update; Senior Fellow & Adjunct Professor, Life Sciences Compliance, Mitchell Hamline School of Law; West Chester, PA |

Ron L. Wisor, Jr, JD
Partner, Hogan Lovells, Washington, DC |

Amy Yuda, MSJ, CHC, CQA, CCEP
Compliance Officer, PolarityTE, Salt Lake City, UT
|
|
|
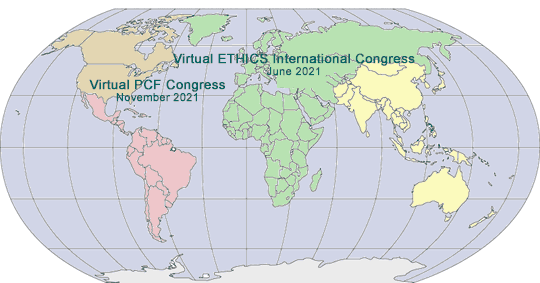
2020-2021 GLOBAL PHARMA/MED DEVICE ETHICS & COMPLIANCE CONGRESSES
|
|
VIRTUAL TWENTY-FIRST ANNUAL PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
|
Virtual Online Video Event Live and Archived
Sponsored by Pharmaceutical Compliance Forum
Media Partners: Harvard Health Policy Review, Health Affairs and Policy & Medicine Compliance Update
November 4 - 6, 2020
www.PharmaCongress.com
|
|
VIRTUAL FOURTEENTH INTERNATIONAL PHARMACEUTICAL AND MEDICAL DEVICE ETHICS & COMPLIANCE CONGRESS
|
Virtual Online Video Event Live and Archived
Sponsored by International Society of Healthcare Compliance Professionals (ETHICS)
Media Partner: Policy & Medicine Compliance Update
February 22 - 25, 2021
www.International PharmaCongress.com
|
|
|
|
|