|
|
|
Virtual ETHICS Int'l Pharma & Med Device Ethics & Compliance Congress Features Annual Medical Device Roundtable
|
-
Virtual Online Video Event Live and 6 Months Access to Video Archive
-
Sponsored by ETHICS, the International Society of Healthcare Compliance Professionals
-
Media Partners: Policy & Medicine Compliance Update
-
June 14-17, 2021
-
www.InternationalPharmaCongress.com
PRESS RELEASE
Phone: 800-503-8171
Email: registration@hcconferences.com
Website:
www.InternationalPharmaCongress.com
|
|
NEW REGISTRATION RATES FOR A VIRTUAL AGE
|
Early bird registration discounts now available with special ETHICS/EFPIA/FSA/IFPMA/MedTech Europe/PCF rates. Rates are up to €1,000 less than major competing events.
- Click Here -
|
|
|
FEATURING THE ANNUAL MEDICAL DEVICE ETHICS & COMPLIANCE ROUNDTABLE
|

Aline Lautenberg, LLM
General Counsel, Director Legal & Compliance, MedTech Europe, Brussels, Belgium
|

Anthony McQuillan, LLB
Vice President Legal & Compliance EMEA, Medtronic International; Former Senior Attorney EMEA, Hewlett-Packard; London, UK
|

Tamara Tubin
International Compliance Director, Corporate Compliance, Wright Medical Group N.V., Vice Chair, Ethics & Compliance Committee, MedTech Europe, Member, Strategic Committee, ETHICS, Zürich, Switzerland
|

Roeland Van Aelst
Regional Lead Third Party Intermediary Ethics & Compliance EMEA, Johnson & Johnson, President, International Society of Healthcare Ethics and Compliance Professionals (ETHICS),Chairman, MedTech Europe Code Committee, Brussels, Belgium
|

Christopher L. White, JD
Chief Operating Officer & General Counsel, Advanced Medical Technology Association (AdvaMed), Washington, DC, USA
|
|
|
PARIS, FRANCE -- PHARMA UPDATE NEWS SERVICE™ -- FEBRUARY 25, 2021: The Virtual Fourteenth International Pharmaceutical and Medical Device Ethics and Compliance Congress,
www.InternationalPharmaCongress.com, has been rescheduled to June 14 - 17, 2021, will take place in the form of online video broadcast which will be offered live and archived for 6 months following the live broadcast.
|
|
KEYNOTE SPEAKERS
|

Hani Abouhalka, MBA
Company Group Chairman, Medical Devices EMEA, Johnson & Johnson, New Brunswick, NJ, USA
|

Gemma Aiolfi
Head of Compliance, Corporate Governance and Collective Action, Basel Institute on Governance; Former Advisor, Working Group on Bribery in International Business Transactions, OECD; Basel, Switzerland
|

Pedro Carrascal, MBA
President, European Multiple Sclerosis Platform (EMSP), Chief Executive Officer, EME (Multiple Sclerosis Spain), Biscay MS Society and MS Basque Foundation, Board, Multiple Sclerosis International Federation (MSIF), Bilbao, Spain
|

Roxana Family, Thèse de Doctorat Droit
Vice-President, Chair in Law & Business Ethics and Program Director, Cergy Pontoise University, Cergy-Pontoise, France
|

Robert I. Dodge, JD
Assistant Director, FCPA Unit, US Securities & Exchange Commission; Former Assistant U.S. Attorney, US Attorney's Office, Western District of Michigan; Former Assistant Section Chief, Environmental Defense Section, US Department of Justice, Washington, DC, USA
|

David Fuhr, JD
Assistant Chief, FCPA Unit, Fraud Section, Criminal Division, US Department of Justice, Washington, DC, USA
|

Kirt Kraeuter, MGA
Senior Director, Corporate Compliance, Moderna; Former European Compliance Director, Otsuka; Former, Executive Director, Worldwide Compliance Committee & Monitoring, Bristol-Myers Squibb; Yardley, PA, USA
|

Sally Molloy, JD
Chief, Strategy, Policy & Training Unit, Fraud Section; Former Assistant U.S. Attorney, US Attorney's Office, Northern District of Georgia, US Department of Justice; Washington, DC, USA
|

Piergiorgio Pepe, MA
EU Law, President, Quantum Ethics, Ethics and Compliance Lecturer, SciencesPo; Former Compliance Director Western Europe & Canada, AbbVie; Former Director, Compliance & Ethics EMEA, Bristol-Myers Squibb; Paris, France
|

N. Craig Smith
Chaired Professor of Ethics and Social Responsibility, Academic Director of ESRI (the INSEAD Ethics and Social Responsibility Initiative), and Programme Director for the Healthcare Compliance, Implementation Leadership Programme, INSEAD, Fontainebleau, France
|
|
|
|
FEATURED FACULTY
|

Imelda Álvarez, LLB, MBA
Chief Executive Officer, Comply Latam, SC; Former Regional Integrity & Compliance Head Latin America and Canada, Novartis; Mexico City, Mexico
|

Ghadeer Alyacoub, MSc
Regional Head Healthcare Compliance Europe, Middle East and Africa Medical Devices, Johnson & Johnson, Dubai, UAE
|

Bas Amesz, MSc
Partner, Vintura, Baarn, Utrecht, Netherlands
|

Michael Bartke, PhD
Director Ethics & Compliance, Alexion, Former Director Compliance Management, Daiichi Sankyo Europe GmbH, Strategic Committee, ETHICS, Munich, Germany
|

Ann Beasley, JD
Chief Compliance Officer, Zai Lab; Former Senior Vice President, Chief Compliance Officer, Biogen; Former Global Compliance Office, Novartis Pharma AG; Boston, MA, USA
|

Duygu Beyazo, LLB
Senior Associate, NSN Law Firm; Former Compliance Manager (Secondment), Business Conduct, Gilead Sciences; Istanbul, Turkey
|

Stefano Biondi, LLB, PhD
Compliance Manager and Group Data Protection Officer, Menarini Group, Florence, Italy
|

Richard Bistrong, MA
Chief Executive Officer, Front-Line Anti-Bribery LLC
Contributing Editor, FCPA Blog; Former Confidential Human Source (CHS) and Cooperating Witness, FBI and US DOJ; New York, NY, USA
|

Eric Bolesh
Chief Operating Officer, Cutting Edge Information, Raleigh-Durham, NC, USA
|

Keith Burn
Global Investigations Director, Ipsen; Former Associate Investigator, Parliamentary and Health Service Ombudsman, NHS; Former Detective Constable, London Metropolitan Police; Slough, UK
|

Zachary N. Coseglia, JD
Managing Principal and Health of Innovation, R&G Insights Lab, Boston, MA
|

Rika De Ville
Vice President Global Compliance & Privacy, Data Protection Officer, Perrigo Company plc Nazareth, Belgium
|

Anisa Dhalla
Global Chief Compliance Officer, UCB, Inc., Atlanta, GA, USA
|

Holger Diener
Healthcare Compliance Officer, Janssen-Cilag GmbH, Johnson & Johnson; Former Managing Director, Association of Voluntary Self-Regulation for the Pharmaceutical Industry (“FSA”);Berlin, Germany
|

Kalwant Dhindsa, CIPP/E, CCEP
Compliance Director, Convatec; Former Senior Corporate Compliance Counsel, Bayer; Reading, UK
|

Laetitia Ducroquet
Vice-President, Global Business Ethics, Ipsen, Boulogne Billancourt, France
|

Julien Durand, MBA, PhD
Vice President, Head of Transformation, Global Ethics & Compliance, Takeda Chair, Future Health Technologies & Bioethics Working Groups, IFPMA; Zürich, Switzerland
|

Signe Elbæk, JD, LLM
Former Group Chief Compliance Officer, Coloplast; Former Vice Chair, Ethics and Compliance Committee and Member of the Code Committee, MedTech Europe; Former Compliance Manager, Attorney, Dako Denmark A/S; Copenhagen, Denmark
|

Jacob Elberg, JD
Associate Professor, Seton Hall University School of Law; Former Chief, Health Care & Government Fraud Unit, and Assistant US Attorney US Attorney's Office, District of New Jersey; Newark, NJ, USA
|

Robert Ennis, JD, LLM
Vice President and Lead Compliance Counsel, Pfizer, New York, NY
|

Alex Fell
Head, Ethics, Compliance and DPO International, Amicus Therapeutics; Former Vice President, Global Ethics and Compliance, Head of Strategy, Planning and Operations, GSK; London, UK
|

George Fife
Partner, Forensic & Integrity Services, EY, Former Executive Director, Compliance & Ethics, Bristol-Myers Squibb, Paris, France
|

Indrani M. Lall Franchini, JD
Executive Vice President & Chief Compliance Officer, Alexion Pharmaceuticals, Inc.; Former Assistant General Counsel, Pfizer; New York, NY, USA
|

Sylvie Gallage-Alwis, DEA, LLM
Partner, Signature Litigation Avocat à la Cour Solicitor in England & Wales Committees Vice Chair, International Association of Defense Counsel, Paris, France
|

Abhiroop Gandhi
Trust and Compliance Officer, Verily Life Sciences (an Alphabet company), Former Vice President, Corporate Compliance, Mallinckrodt, San Francisco, CA, USA
|

Gary F. Giampetruzzi, Esq.
Partner, Paul Hastings, Former Vice President and Assistant General Counsel, Head of Government Investigations, Pfizer Inc., New York, NY, USA
|

Tom Gregory
Partner, Fraud Investigation & Dispute Services, Ernst & Young, LLP, Atlanta, GA
|

Ulf H. Grundmann
Partner, FDA and Life Sciences, King & Spalding LLP, Frankfurt am Main, Germany
|

Joseph W. Henein, PharmD
President and Chief Executive Officer, NewBridge Pharmaceuticals, Dubai, UAE
|

Bella Rafael Hovhannisyan
Acting Chief Compliance Officer, Business Integrity Lead, Intercontinental, CSL Bering; Former Compliance & Ethics Lead, Spain & Portugal, Russia & Turkey, Bristol-Myers Squibb; Berne, Switzerland
|

Mirgen Jaku, MA
Lead of Market Readiness, Digital Surgery, EMEA, Johnson & Johnson, Hamburg, Germany
|

Franziska Janorschke
Global Head, Business Practices Office, Novartis International AG, Basel, Switzerland
|

Els Janssens, LLM
Associate, Baker & McKenzie; Former Legal Adviser, European Medicines Agency; Former Senior Legal Counsel, Johnson & Johnson; Brussels, Belgium
|

Larisa K. Jasnic, LLB, LLM
Head of Ethics & Compliance, International, Alnylam Pharmaceuticals; Former Regional Compliance Officer, Region Europe, Novartis; Zug, Switzerland
|

Elisabeth Kohoutek
Senior Associate, FDA and Life Sciences, King & Spalding LLP, Frankfurt am Main, Germany
|

Keith Korenchuk, MPH, JD
Vice President and Chief Compliance Officer, DH Diagnostics, a Danaher Company, Chevy Chase, MD, USA
|

Iordanis Kerenidis, PhD
Director, Paris Centre for Quantum Computing (PCQC) CNRS Senior Researcher (DR2), Algorithms and Complexity Group IRIF, University Paris Diderot, Paris, France
|

Adem Koyuncu, MD, PhD (Law)
Partner and Chair, Food, Drug & Device Practice Group, Covington; Brussels, Belgium & Frankfurt, Germany
|

Tomasz Kruk
Head of Compliance, Vifor Pharma, Former Director International Compliance, Mallinckrodt Pharmaceuticals; Former Director Global Ethics & Compliance, Actavis plc (now Allergan); Zurich, Switzerland
|

Aline Lautenberg, LLM
General Counsel, Director Legal & Compliance, MedTech Europe, Brussels, Belgium
|

Lei Li, LLM
Managing Partner, Beijing and Shanghai Offices, Sidley Austin; Former Third Secretary, Ministry of Commmerce, People's Republic of China, Beijing, China; Beijing, China
|

Rosa Magistri, CIPP/E
Head of Legal and Compliance Europe, SeaGen International GmbH; Former Regional Director Office of Ethics and Compliance, Western Europe & Canada, Region South & Israel, AbbVie; Former Associate Compliance Director, Shire; Zug, Switzerland
|

Sofie Melis
Director HR and Ethics & Compliance, International Federation of Pharmaceutical Manufacturers and Associations (IFPMA), Geneva, Switzerland
|

Anthony McQuillan, LLB
Vice President Legal & Compliance EMEA, Medtronic International; Former Senior Attorney EMEA, Hewlett-Packard; London, UK
|

Philippa Montgomerie, CPE, LLB
Vice President EMEA Compliance and Global Programs, Medtronic Limited, Watford, UK
|

Arthur Muratyan, Esq.
Secretary General, International Society of HealthCare, Ethics and Compliance Professionals (ETHICS), Chair, MedTech Compliance Panel; Former VP-Head of Legal Corporate and Global Compliance Officer, Sanofi
|

Laura Nassar, PharmD
Head of Compliance Middle East, Roche Pharmaceuticals, Chair, Compliance Committee, Pharmaceutical Research, and Manufacturers Association, Gulf (PhRMAG), Beirut, Lebanon
|

Gina Nese, JD
Vice President Global Compliance and Ethics Officer, Align Technology; Former Chief Compliance Officer, Advanced Sterilization Products; Bellevue, WA, USA
|

Lauri Q. Opar
Head, Enterprise Risk Management & Risk Analytics, Global Ethics & Compliance, Glaxosmithkline, Brentford, Middlesex, UK
|

Giota Papamarkou
Vice-President, Business Ethics Global, France, Ipsen; Former EMEA Compliance and Ethics Manager, Bristol-Myers Squibb; Paris, France
|

Mario Prohasky
Principal, EMEA Compliance Consulting, IQVIA, London, UK
|

Ariadna Quesada, MSJ
Compliance Director Europe and MEATI, Hillrom; Former Law, Ethics and Compliance Officer, The Netherlands, AbbVie; Former Compliance Manager International, MicroPort Orthopedics; Amsterdam, Netherlands
|

Amanda N. Raad, JD
Co-lead, Anti-Corruption and International Risk, Ropes & Gray, London, UK
|

Nadege Rochel
Global Compliance Manager, Holister Incorporated, Milan, Italy
|

Christian-Claus Roth
Head Scientific Engagement Governance, Novartis, Co-president, International Pharmaceutical Congress Advisory Association (IPCAA), Member, Ethics and Business Integrity Committee, IFPMA, Basel, Switzerland
|

Giuseppe Saporito, LLM
Global Legal & Regulatory Expert, IQVIA, Milan, Italy
|

Leonardo Silva, LLM
Global Compliance Officer, Head of Global Compliance, Ferring, Former Head of Compliance, Acino Group, Former Compliance Director, Emerging Markets, Takeda, St. Prex, Switzerland
|

Tiffany Tang
Associate Principal, Global Consulting, IQVIA; Former Senior Manager, Ethics and Compliance, Novo Nordisk; New York, NY
|

Madina Torchinova
Vice President, Head Global Compliance, MorphoSys; Former Regional Head of Compliance, Central & Eastern Europe, Middle East & Africa, and Global OTC, Sandoz; Munich, Germany
|

Tamara Tubin
International Compliance Director, Corporate Compliance, Wright Medical Group N.V., Vice Chair, Ethics & Compliance Committee, MedTech Europe, Member, Strategic Committee, ETHICS, Zürich, Switzerland
|

Samar Wakim, PharmD
Ethics & Compliance Head MEA, Innovative Medicines, Novartis Pharma Services AG, Dubai, UAE
|

Christopher L. White, JD
Chief Operating Officer & General Counsel, Advanced Medical Technology Association (AdvaMed), Washington, DC, USA
|

Mariusz Witalis
Partner, Forensic & Integrity Services, EY, Warsaw, Poland
|
|
|
|
|
|
CO CHAIRS
|

Anne-Sophie Bricca, MA, DEA International Law, DES European law
Deputy General Counsel & Senior Director Legal Affairs & Compliance, Terumo BCT; Chair, Ethics and Compliance Group and
seats at the Code Committee, MedTech Europe; Brussels, Belgium
|

Stephen Nguyen Duc
Vice-President, Global Head of Human Resources and Ethics & Compliance, Medday Pharmaceuticals; Board Member, Strategic Committee International Society of Healthcare
Ethics and Compliance Professionals (ETHICS); Paris, France
|

Dominique Laymand, Esq.
Executive Vice President, Chief Ethics & Social Responsibility Officer, Ipsen; Honorary President, International Society of Healthcare Ethics and Compliance Professionals
(ETHICS); Paris, France
|

Roeland Van Aelst
Regional Lead Third Party Intermediary Ethics & Compliance EMEA, Johnson & Johnson, President, International Society of Healthcare Ethics and Compliance Professionals
(ETHICS),Chairman, MedTech Europe Code Committee, Brussels, Belgium
|
|
SPONSORED BY:
|

|
|
GRANTORS
|
|
GOLD
|

|
|
SILVER
|


|
|
BRONZE
|



|
|
MEDIA PARTNER:
|

|
|
STAY CONNECTED
|


Tweet using #PharmaCongress
|
|
CONGRESS HIGHLIGHTS
|
-
New content, optimized for online learning, focused on new regulatory developments and managing ethics and compliance programs during the COVID
19 pandemic
-
Engage with faculty through polling, Q&A sessions, chat and direct video messaging
-
Network in real time with other attendees through direct attendee to attendee and group video messaging
-
Live and curated sessions streamed directly to you through our easy to navigate virtual platform
-
Access to an extensive video archive of past Congress presentations providing a context for the evolution of the
compliance professions and the legal and regulatory setting
-
Virtual exhibit hall featuring innovative solutions, products and services with opportunity to directly
contact exhibitors for further information
|
|
|
|
|
PAST GLOBAL PHARMA COMPLIANCE CONGRESSES
|

2007 - Brussels

2008 - Paris

2009 - Rome

2010 - Berlin

2011 - Istanbul

2011 - Singapore

2012 - Budapest

2012 - Shanghai

2012 - São Paulo

2013 - Madrid

2013 - Kuala Lumpur

2014 - Dubai

2014 - Mexico City

2014 - Shanghai

2015 - Manila

2016 - Warsaw

2017 - Lisbon

2018 - Vienna

2019 - Athens
|
|
|
FEATURING THE INTERNATIONAL CONGRESS ADVANCED VIRTUAL STREAMING PLATFORM
|

|
|
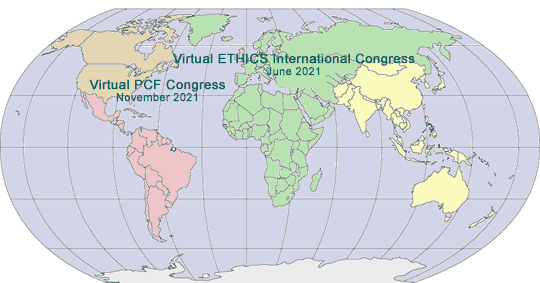
2021 GLOBAL PHARMA/MED DEVICE ETHICS & COMPLIANCE CONGRESSES
|
|
VIRTUAL FOURTEENTH INTERNATIONAL PHARMACEUTICAL AND MEDICAL DEVICE ETHICS & COMPLIANCE CONGRESS
|
Virtual Online Video Event Live and Archived
Sponsored by International Society of Healthcare Compliance Professionals (ETHICS)
Media Partner:
Policy & Medicine Compliance Update
June 14-17, 2021
www.International PharmaCongress.com
|
|
VIRTUAL TWENTY-SECOND ANNUAL PHARMACEUTICAL AND MEDICAL DEVICE ETHICS & COMPLIANCE CONGRESS
|
Virtual Online Video Event Live and Archived
Sponsored by Pharmaceutical Compliance Forum
Media Partners:
Harvard Health Policy Review, Health Affairs and
Policy & Medicine Compliance Update
November, 2021
www.PharmaCongress.com
|
|
|
|
CONGRESS EXHIBIT & SPONSORSHIP INFORMATION
|
|
For sponsorship and exhibit information, visit or contact Suzanne Tyler, Exhibit Manager, at (206) 244-4861 phone, (206) 319-5303 fax, or
exhibits@hcconferences.com.
|
|
|
|
|