PRESS RELEASE: The Pharmaceutical Compliance Forum's Thirteenth Pharmaceutical Regulatory Compliance Congress and Best Practices Forum Features Global R&D and Medical Affairs Compliance Issues

- Sponsored by the Pharmaceutical Compliance Forum
- Onsite at Grand Hyatt, Washington, DC
- Online in Your Own Office or Home Live via the Internet with 24/7 Access for Six Months
- November 5 - 7, 2012
- Grand Hyatt, Washington, DC
- www.PharmaCongress.com
PRESS RELEASE
Phone: 800-503-7419
Email: registration@hcconferences.com
Website: www.PharmaCongress.com
|
|
WASHINGTON DC USA -- PHARMA UPDATE NEWS SERVICE™ -- SEPTEMBER 27, 2012: The Thirteenth Annual Pharmaceutical Regulatory and Compliance Congress and Best Practices Forum (the Pharma Congress), www.PharmaCongress.com, will take place on November 5 - 7, 2012 at the Grand Hyatt in Washington, DC. Registrants may attend the event in person or online (live webcast and archived for 6 months).
|
FEATURING COMPLIANCE ISSUES IN GLOBAL R&D AND MEDICAL AFFAIRS
|
- Regulatory Enforcement Environment: Has the Bar Moved?
- Applying Quality Management Principles to R&D
- Managing Global Trials -- Special Considerations
- Publication of Clinical Trial Results
- Support of Medical Education Globally
- Compliance Challenges with Investigator-Sponsored Research

Stuart Horowitz, PhD, MBA
President, Institutions & Institutional Services, Arsenal WGH Holdings, Inc., Western Institutional Review Board, Olympia, WA |

Gerald "Jerry" Kuncio, PhD
Deputy Compliance Officer for North America Medical Affairs, GlaxoSmithKline; Former Medical/Scientific Compliance and Ethics Director, AstraZeneca, Philadelphia, PA |

Ann Meeker-O'Connell, MS, CCEP
Office of Policy, Office of the Commissioner, US Food and Drug Administration, Silver Spring, MD
|

Mark Barnes
Director of Sponsored Research and Clinical Research Compliance Officer, Harvard University, Cambridge, MA (Co chair) |

Kathleen Meriwether, Esq.
Principal, Fraud Investigation and Dispute Services, Ernst & Young LLP; Philadelphia, PA (Co chair)
|
|
|
|
KEYNOTE SPEAKERS
|

Thomas W. Abrams, RPh, MBA
Director, Office of Prescription Drug Promotion (OPDP), US Food and Drug Administration, Silver Spring, MD |

Lanny A. Breuer, Esq.
Head, Criminal Division, US Department of Justice, Former Special White House Counsel, Former Assistant District Attorney, New York City, Washington, DC |

Deirdre Connelly
President - North America Pharmaceuticals, GlaxoSmithKline, Former President of US Operations, Eli Lilly and Company, Philadelphia, PA
|

Gregory E. Demske, Esq.
Chief Counsel to the Inspector General, Office of Inspector General, Department of Health and Human Services, Washington, DC |

Susan Dentzer
Editor-in-Chief, Health Affairs, Health Policy Analyst, The News Hour with Jim Lehrer, Washington, DC |

Louis Joseph Freeh, JD, LLM
Partner, Pepper Hamilton LLP; Founder and Chairman, Freeh Group International Solutions; Former Director, Federal Bureau of Investigation; Former Judge, United States District Court, Southern District of New York, Wilmington, DE
|

Paul E. Kalb, JD, MD
Partner and Global Coordinator, Life Sciences Practice, Sidley Austin LLP, Washington, DC |

Carmen M. Ortiz, Esq.
United States Attorney, District of Massachusetts, Boston, MA |

Mary E. Riordan, Esq.
Senior Counsel, Office of Counsel to the Inspector General, Office of Inspector General, Department of Health and Human Services, Washington, DC
|
|
|
|
FEATURING PRECONFERENCE SESSIONS
|
- Compliance 101
- Auditing and Monitoring Boot Camp
- A Comprehensive Overview of Pharma and Medical Device Corporate Integrity Agreements (CIAs)
- The New Era of Scrutiny: FCPA Compliance in Pharma Operations
|
|
|
|
PLENARY SESSIONS
|
- OIG Update
- DOJ Criminal Division Update
- Prosecuting Pharma and Device Fraud
- FDA-DDMAC Update
- AUSA Panel
- Qui Tam Panel
- State Enforcement Panel
- Best Practices in Negotiating and Implementing CIAs
- State Disclosure, Federal Sunshine Act and Global Transparency
- Life Sciences in America the Morning after the Election
- Managing Internal and External Investigations
- PhRMA's New Compliance Work Group Update
- Global Pharma and Device Compliance Issues and Strategies
|
|
PHARMA CONGRESS IS
|

|
|
|
|
AND MINI SUMMITS
|
- Mini Summit I: Co-pay Coupon Litigation Update
- Mini Summit II: What Enhanced Obligations in CIAs and DPAs say about Agency Expectations for Compliance Programs
- Mini Summit III: Compliance Issues in Global R&D and Medical Affairs
- Mini Summit IV: US Disclosure Implementation Update
- Mini Summit V: Medical Device Compliance Issues Update
- Mini Summit VI: Global Pharma and Device Compliance Issues
- Mini Summit VII: Anticorruption, Including FCPA and UK Bribery Act Update
- Mini Summit VIII: Fair Market Value Update
- Mini Summit IX: Enforcement Threat Against Individuals
- Mini Summit X: Global Transparency Update
- Mini Summit XI: Special Compliance Issues and of Small Pharma and Medical Device Companies
- Mini Summit XII: Integrating a Culture of Ethics into Your Compliance Program
- Mini Summit XIII: Government Price Reporting Update
- Mini Summit XIV: Clinical Trial Disclosure and Results Reporting Liability under FDAAA, Section 801
- Mini Summit XV: Board and Management Certifications and Working with an IRO
- Mini Summit XVI: Drug Samples Disclosure: The Next Horizon?
- Mini Summit XVII: Professional Responsibilities for Compliance Officers and In-house Counsel in the
- Pharmaceutical, Biotech and Medical Device Industries
- Mini Summit XVIII: Compliance Program Innovation
|
|
|
LEADING ASSISTANT US ATTORNEYS
|

Paul Kaufman, Esq.
Assistant US Attorney and Chief, Civil Health Care Fraud, United States Attorney's Office, Eastern District of New York, Brooklyn, NY |

Marilyn May, Esq.
Senior Litigation Counsel, US Attorney's Office, Eastern District of Pennsylvania, United States Department of Justice, Philadelphia, PA |

Maureen Ruane, Esq.
Assistant US Attorney and Chief, Health Care and Government Fraud Unit, Criminal Division, United States, Attorney's Office, District of New Jersey, Newark, NJ
|

Susan Winkler, Esq.
Assistant US Attorney, United States Attorney's Office, District of Massachusetts, Boston, MA |

John T. Bentivoglio, Esq.
Partner, Skadden Arps LLP; Former Special Counsel for Healthcare Fraud and Chief Privacy Officer, US Department of Justice, Washington, DC (Moderator)
|
|
|
|
LEADING STATE PROSECUTORS
|

Jacob Bergman, Esq. (Invited)
Special Assistant Attorney General, Medicaid Fraud Control Unit, NY Office of the Attorney General, New York, NY |

Keesha Mitchell, Esq.
Chief, Health Care Fraud Section, Director, Medicaid Fraud Control Unit, Ohio Attorney General's, Office, Columbus, OH |

Cynthia O'Keeffe
Deputy Chief, Civil Medicaid Fraud Division, Texas Office of the Attorney General, Austin, TX
|

Nicholas N. Paul, Esq.
Supervising Deputy Attorney, General, Bureau of Medi-Cal Fraud and Elder Abuse, Office of the Attorney General, California Department of Justice San Diego, CA |

Virginia "Ginny" A. Gibson, Esq.
Partner, Hogan Lovells, US LLP; Former Executive Assistant US Attorney, United States, Attorney's Office, Eastern District of Pennsylvania, Philadelphia, PA (Moderator)
|
|
|
|
LEADING QUI TAM COUNSEL
|

Erika A. Kelton, Esq.
Partner, Phillips & Cohen LLP, Washington, DC |

Daniel R. Miller, Esq.
Partner, Berger & Montague, PC, Former Deputy Attorney General, Delaware Department of Justice, Philadelphia, PA |

Michael A. Morse, Esq.
Partner, Pietragallo Gordon Alfano Bosick & Raspanti, LLP, Former Assistant District Attorney, Philadelphia District Attorney's Office, Philadelphia, PA |

Joseph E. B. "Jeb" White, Esq.
Partner, Nolan & Auerbach, PA, Philadelphia, PA |

Kirk Ogrosky, Esq.
Partner, Arnold & Porter, Former Deputy Chief, Fraud Section, US Department of Justice, Washington, DC (Moderator)
|
|
|
|
REPORT FROM THE NEW PhRMA COMPLIANCE WORK GROUP
|

Kendra Martello, Esq.
Assistant General Counsel, PhRMA, Washington, DC |

Anne Nobles, MA, JD
Chief Ethics and Compliance Officer and Senior Vice President Enterprise Risk Management, Eli Lilly and Company, Indianapolis, IN
|
|
|
|
COMPLIANCE 101
|

Gary DelVecchio
Executive Director, US Pharmaceutical Compliance and Ethics, Bristol-Myers Squibb Company, Plainsboro, NJ |

Margaret K. Feltz, Esq.
Director, Corporate Compliance, Purdue Pharma LP, Stamford, CT |

Michael Kendall, Esq.
Partner and Head, White-Collar Defense Group, McDermott Will & Emery LLP, Former Deputy Associate Attorney General and Counselor, United States Department of Justice, Boston, MA
|

Janet L. "Lucy" Rose
Founder, Lucy Rose and Associates, Former Director, Division of Drug Marketing, Advertising, and Communications (DDMAC), FDA, Washington, DC |

Kelly B. Freeman, PhD
Ethics and Compliance Officer, Eli Lilly and Company, Indianapolis, IN (Moderator)
|
|
|
|
DRUG SAMPLES DISCLOSURE: THE NEXT HORIZON?
|

Kendra Martello, Esq.
Assistant General Counsel, PhRMA, Washington, DC |

Marilyn May, Esq.
Senior Litigation Counsel, US Attorney's Office, Eastern District of Pennsylvania, United States Department of Justice, Philadelphia, PA |

Kate Whelley McCabe, Esq. (Invited)
Assistant Attorney General, Public Protection Division, Vermont Office of the Attorney General, Montpelier, VT
|
Karen Rothschild, Esq. (Invited)
Regulatory Counsel, Office of Compliance, Center for Drug Evaluation and Research, Food and Drug Administration, Washington, DC |

John Patrick Oroho, Esq.
Executive Vice President and Chief Strategy Officer, Porzio Pharmaceutical Services, LLC, Principal, Porzio, Bromberg & Newman PC, Morristown, NJ (Moderator)
|
|
|
|
INTEGRATING A CULTURE OF ETHICS INTO YOUR COMPLIANCE PROGRAM
|

Paul J. McNulty, Esq.
Partner and Chairs, Global Corporate Compliance Steering Committee, Baker & McKenzie LLP, Former Deputy Attorney General, US department of Justice, Washington, DC |

Matthew Pachman, Esq.
Vice President and Chief Ethics Officers, FTI Consulting, VP, Chief Compliance Officer, Altegrity, VP, Compliance, Ethics and Business Practices, Freddie Mac, Director, Legal (and Ethics), MCI, Washington, DC
|

Jeffrey S. Paden
Deputy Compliance Officer, GlaxoSmithKline, Research Triangle Park, NC |

Caroline West, Esq.
Senior Vice President, Chief Compliance and Risk Officer, Shire Pharmaceuticals, Inc., Philadelphia, PA (Moderator)
|
|
|
|
ANTICORRUPTION UPDATE
|

Gary F. Giampetruzzi, Esq.
Vice President and Assistant General Counsel, Head of Government Investigations, Pfizer Inc., New York, NY |

Michael Kendall, Esq.
Partner and Head, White-Collar Defense Group, McDermott Will & Emery LLP, Former Deputy Associate Attorney General and Counselor, United States Department of Justice, Boston, MA |

Vivian Robinson, Esq.
Partner, McGuireWoods, Former General Counsel of the UK Serious Fraud Office Former Head, QEB Hollis Whiteman Chambers, Recorder of the Crown Court and Treasurer of Inner Temple, London, UK
|
|
|
|
THE NEW ERA OF SCRUTINY: FCPA COMPLIANCE IN PHARMA OPERATIONS
|

Gregory A. Paw, Esq.
Partner, Pepper Hamilton LLP, Former Director, Division of Criminal Justice, Office of the New Jersey Attorney General, Former Deputy US Attorney, US Attorney's Office for the Eastern District of Pennsylvania, Philadelphia, PA |

John G. Rahie, JD, LLM
Partner, Freeh Sporkin & Sullivan, LLP, Former Executive Director of Global Investigations, General Motors Corporation, Washington, DC
|
|
|
|
ENFORCEMENT THREAT AGAINST INDIVIDUALS
|

Thomas M. Gallagher, Esq.
Chair, White Collar Litigation and Investigations Practice Group, Pepper Hamilton LLP, Philadelphia, PA |

Lori Queisser
Principal, Advisory Services, KPMG LLP, Former Senior Vice President, Global Compliance, and Business Practices, Schering-Plough Corporation, Former Member, PCF Executive Committee, Indianapolis, IN
|
|
|
|
BEST PRACTICES IN NEGOTIATING AND IMPLEMENTING A CIA
|

Cynthia Cetani
Vice President, Ethics & Compliance, Chief Compliance Officer, Novartis Pharmaceuticals Corporation, New York, NY |

Lauran S. D'Alessio
Vice President and Compliance Officer, Merck & Co., Whitehouse Station, NJ |

Kris Curry
Vice President, Health Care Compliance, Johnson & Johnson Pharmaceuticals, Titusville, NJ
|

Michael L. Shaw
Vice President & Compliance Officer, GlaxoSmithKline-North America Pharmaceuticals, Former Senior Counsel, Office of Inspector General, Philadelphia, PA |

Thomas A. Gregory, CFA, MBA
Partner, Fraud Investigation & Dispute Services, Ernst & Young LLP, Atlanta, GA (Moderator)
|
|
|
A COMPREHENSIVE OVERVIEW OF PHARMA AND MEDICAL DEVICE
CORPORATE INTEGRITY AGREEMENTS (CIAS)
|

Tracy Mastro, MBA
Senior Director, Life Sciences Advisory Services, Huron Consulting Group, Washington, DC |
Paige Slater, PA-C
Global CIA Program Director, Corporate Ethics and Compliance, Synthes, Inc., West Chester, PA |

Bert Weinstein, Esq.
Vice President, Corporate Compliance, Purdue Pharma LLP; Former Member, PCF Executive Committee, Stamford, CT
|

Wendy C. Goldstein, Esq.
Partner, Epstein Becker & Green, New York, NY (Moderator) |

Paul J. Silver
Practice Leader, Life Sciences Advisory Services, Huron Consulting Group, Atlanta, GA (Moderator)
|
|
|
WHAT ENHANCED OBLIGATIONS IN CIAs AND DPAs SAY ABOUT
AGENCY EXPECTATIONS FOR COMPLIANCE PROGRAMS
|

Steve Guymon
US Medical Compliance Officer, Eli Lilly and Company, Indianapolis, IN |

Steven J. Tave, Esq.
Counsel, Gibson, Dunn & Crutcher LLP; Former Associate Chief Counsel for Enforcement, Office of Chief Counsel, US Food and Drug Administration, Washington, DC
|

Thomas W. Beimers, Esq.
Special Counsel, Faegre Baker Daniels; Former Senior Counsel for Administrative and Civil Remedies, Office of the Inspector General, US Department of Health and Human Services, Minneapolis, MN (Moderator) |

Edward Nowicki, Esq.
Vice President and Assistant General Counsel, Pfizer Inc., New York, NY (Moderator)
|
|
|
|
BOARD AND MANAGEMENT CERTIFICATIONS AND WORKING WITH AN IRO
|

Victoria Browning
Senior Director Corporate Compliance Operations, Allergan Inc., Irvine, CA |

Daniel J. Garen, Esq.
Senior Vice President and Chief Compliance Officer, Wright Medical Technology, Inc., Former Chief Compliance Officer and Senior Counsel, Siemens Healthcare Sector, Arlington, TN
|

Meredith Manning, Esq.
Co-director, Pharmaceutical and Biotechnology, Practice Group, Hogan Lovells LLP, Former Assistant US Attorney, Civil Division, US Attorney's Office in Washington, DC, Former Associate Chief Counsel, Office of General Counsel, FDA, Washington, DC |

Jean McKiernan
Director, Advisory Pharmaceutical and Life Sciences, PwC Chicago, IL
|
|
|
STATE DISCLOSURE LAWS, SUNSHINE ACT AND
GLOBAL TRANSPARENCY INITIATIVES
|

Niall Brennan, MPP (Invited)
Acting Director, Policy and Data Analysis Group, Centers for Medicare and Medicaid Services, Washington, DC |

William E. Buzzeo, MS
Vice President and General Manager, Compliance Solutions Division, Cegedim Relationship Management, Richmond, VA |

Katrina S. Cahill
Senior Manager, Global Transparency Lead, Biogen Idec, Weston, MA
|

Trudy J. Seeley
Senior Manager, Transparency Operations, Sanofi US, Bridgewater, NJ |

Jonathon Kellerman
Principal, Pharmaceutical and Life Sciences, Advisory Services, PwC, Florham Park, NJ (Moderator)
|
|
|
|
US DISCLOSURE IMPLEMENTATION UPDATE
|

Daniel Char, Esq.
Associate General Counsel - Commercial, Smith & Nephew, Boston, MA |

Diane Cruz-Burke, Esq.
Assistant General Counsel, Eli Lilly and Company, Indianapolis, IN |

Gus Papandrikos, MBA
Director Transparency Operations, Sanofi, New York, NY
|

Eve M. Brunts, JD, LLM
Partner, Ropes & Gray, Boston, MA (Moderator) |

Jack T. Tanselle
Director, Healthcare Dispute, Compliance and Investigation Practice, Navigant Consulting, Inc., Indianapolis, IN (Moderator)
|
|
|
|
GLOBAL TRANSPARENCY UPDATE
|

Peter Burberry
Senior Director, Global Practices, Business Practice Management, Allergan Inc., Irvine, CA |

Michael O'Connor, MS
Executive Director, IS Business Consulting, Boehringer Ingelheim, New York, NY |

Katrina S. Cahill
Senior Manager, Corporate Compliance - Global Transparency Lead, Biogen Idec, Weston, MA
|

William E. Buzzeo, MS
Vice President and General Manager, Compliance Solutions Division, Cegedim Relationship Management, Richmond, VA (Moderator) |

Kelly N. "Nikki" Reeves, MPA, JD
Partner, King & Spalding LLP, Washington, DC (Moderator)
|
|
|
PROFESSIONAL RESPONSIBILITY FOR PHARMA, DEVICE AND BIOTECH
IN-HOUSE COUNSEL AND COMPLIANCE PROFESSIONALS
|

Edward (Ed) Berg, Esq.
Vice President, Associate General Counsel, Sanofi, New York, NY |

Robert Hoehn, Esq.
Health Care Compliance Officer, Acclarent (a Johnson & Johnson company), San Francisco, CA |

Freddy Jimenez, Esq.
Assistant General Counsel, Johnson & Johnson, New Brunswick, NJ
|

Jeffrey Klimaski, CPA
Vice President, Corporate Ethics & Compliance Officer, BTG International Inc., Philadelphia, PA |

Thomas E. Costa
Vice President, US Pharmaceuticals Compliance, Bristol-Myers Squibb Co., Princeton, NJ |

Christopher D. Zalesky, JD, CCEP, RAC
Vice President Global Policy and Guidance, Johnson & Johnson Health Care Compliance and Privacy, New Brunswick, NJ (Moderator)
|
|
|
|
AUDITING AND MONITORING BOOT CAMP
|

Lori Alarimo, Esq.
Vice President and Deputy Compliance Officer, Allergan, Former Assistant General Counsel, Pfizer, Irvine, CA |

Thomas C. Frongillo, Esq.
Partner, Head of Litigation (Boston Office) and Co-Chair, White Collar Criminal Practice, Weil Gotshal & Manges, Boston, MA |

Jeffrey L. Handwerker, Esq.
Partner, Arnold & Porter LLP, Washington, DC
|

Michael Hercz, Esq.
Director, Audit and Enterprise Risk Services, Deloitte & Touche LLP; Former Vice President and Chief Compliance Officer, Victory Pharmaceuticals, Inc., Costa Mesa, CA |

Vickie L. McCormick
Vice President, Health Care Compliance, DePuy, Inc.; Chief Compliance Officer, DePuy Orthopaedics, Inc.; Former Chief Compliance Officer, St. Jude Medical, Warsaw, IN
|

Jeff Rosenbaum
Vice President, Chief Compliance Officer, Vertex Pharmaceuticals; Former Global Head, Ethics & Compliance, Novartis Oncology, Boston, MA |

L. Stephan Vincze, JD, LL M, MBA
Director, Audit and Enterprise, Risk Services, Deloitte & Touche LLP; Former Vice President, Ethics and Compliance Officer/Privacy Officer, TAP Pharmaceutical Products Inc., Boston, MA (Moderator)
|
|
|
|
GOVERNMENT PRICE REPORTING UPDATE
|

George Kenny, Esq.
Senior Account Manager, 340B, Genentech, San Francisco, CA |

Cambria Smith, Esq.
Senior Corporate Counsel, Healthcare Law Group, Genentech, Former Senior Counsel, Amgen, San Francisco, CA
|

Marcy Imada
Principal, Deloitte & Touche LLP, Los Angeles, CA (Moderator) |

John D. Shakow, Esq.
Partner, FDA & Life Sciences Practice, King & Spalding, Washington, DC (Moderator)
|
|
|
|
CO-PAY COUPON LITIGATION UPDATE
|

Perry Goldman, Esq.
Vice President and Deputy General Counsel, Onyx Pharmaceuticals, San Francisco, CA |

William A. Sarraille, Esq.
Partner, Sidley Austin LLP, Washington, DC |

Richard L. Zimmerer
Partner, Forensic Advisory Services, KPMG LLP, Los Angeles, CA
|
|
|
|
COMPLIANCE EFFECTIVNESS REVIEWS: BENEFITS TO THE BOARD OF DIRECTORS AND BEYOND
|

Joseph Cacciatore, MBA
Executive Director, Ethics & Compliance, Novartis Pharmaceuticals Corporation, East Hanover, NJ |

Saul B. Helman, MD, MBA
Managing Director, Disputes and Investigations Practice, Navigant, Chicago, IL
|
|
|
|
FAIR MARKET VALUE UPDATE
|

Mark A. DeWyngaert, PhD
Managing Director, Huron Consulting Group, LLC, New York, NY |

Fred Eaton, MBA
Partner, Polaris Management Partners, New York, NY
|

Prateep Menon, CFA
Principal, Deloitte Financial Advisory Services LLP, New York, NY |

John Moose, MBA, CPA, ABV
Manager, Huron LifeSciences, Chicago, IL
|
|
|
|
COMPLIANCE ISSUES IN GLOBAL R&D AND MEDICAL AFFAIRS
|

Stuart Horowitz, PhD, MBA
President, Institutions & Institutional Services, Arsenal WGH Holdings, Inc., Western Institutional Review Board, Olympia, WA |

Gerald "Jerry" Kuncio, PhD
Deputy Compliance Officer for North America Medical Affairs, GlaxoSmithKline; Former Medical/Scientific Compliance and Ethics Director, AstraZeneca, Philadelphia, PA
|

Mark Barnes
Director of Sponsored Research and Clinical Research Compliance Officer, Harvard University, Cambridge, MA (Moderator) |

Kathleen Meriwether, Esq.
Principal, Fraud Investigation and Dispute Services, Ernst & Young LLP; Philadelphia, PA (Moderator)
|
|
|
|
CLINICAL TRIAL DISCLOSURE AND RESULTS REPORTING LIABILITY UNDER FDAAA, SECTION 801
|

Jeffrey K. Francer, MPP, JD
Assistant General Counsel, PhRMA; Former Associate Chief Counsel, US Food and Drug Administration, Washington, DC, USA |

Ann Meeker-O'Connell, MS, CCEP
Office of Policy, Office of the Commissioner, US Food and Drug Administration, Silver Spring, MD
|

Marc B. Wilenzick, Esq.
Compliance Officer/Chief Compliance Counsel, R&D and Medical, Pfizer Inc., New York, NY |
Julie Finegan, Esq.
Associate Chief Counsel, US Food and Drug Administration, Silver Spring, MD (Moderator)
|
|
|
SPECIAL COMPLIANCE ISSUES AND STRATEGIES OF SMALL
PHARMACEUTICAL AND MEDICAL DEVICE COMPANIES
|

Justin A. Dillon
Vice President, Chief Ethics and Compliance Officer, Ipsen Biopharmaceuticals, Inc.; Former Deputy Ethics and Compliance Officer, North America Pharma and Vaccines, GlaxoSmithKline, Basking Ridge, NJ |

Jeffrey Klimaski, MBA, CPA
Vice President, Corporate Ethics and Compliance Officer, BTG International Inc.; Former Vice President, Global Ethics and Compliance Officer, Stiefel Laboratories, Inc., West Conshohocken, PA |

Daniel A. Kracov, Esq.
Partner and Head, FDA and Healthcare Practice, Arnold & Porter, Washington, DC
|

Timothy Ayers, JD, MPH
Vice President and Chief Compliance Officer, Dendreon; Former Associate General Counsel and Executive Director of Compliance, Seattle Genetics, Bothell, WA (Moderator) |

Elizabeth V. Jobes, Esq.
Senior Vice President and Chief Compliance Officer, Auxilium Pharmaceuticals Inc; Former Vice President and Chief Compliance Officer, Adolor, Philadelphia, PA (Moderator)
|
|
|
|
MEDICAL DEVICE COMPLIANCE ISSUES UPDATE
|

Eileen Erdos
Principal, Fraud Investigation and Dispute Services, Ernst & Young LLP, Chicago, IL, USA |

J. J. Kuhn, Esq.
Senior Director, Ethics and Compliance, Medtronic Neuromodulation, Minneapolis, MN
|

Sujata T. Dayal, Esq.
Corporate Vice President and Chief Compliance Officer, Biomet, Former Counsel, Domestic, Legal Operations, Abbott Laboratories, Former Member, PCF Executive Committee, Warsaw, IN (Moderator) |

Ronald L. Wisor, Jr., Esq.
Partner, Hogan Lovells US LLP, Washington, DC (Moderator)
|
|
|
|
GLOBAL PHARMA AND DEVICE COMPLIANCE ISSUES AND STRATEGIES
|

Sue Egan
Director and Principal Consultant, Sue Egan Associates, Editor, Life Science Compliance, Former Vice President Compliance, AstraZeneca, Great Missenden, Buckinghamshire, UK |

Keith M. Korenchuk, JD, MPH
Partner, Arnold & Porter LLP, Washington, DC |

Abdul Luheshi, MBA, PhD
Vice President Health Care Compliance, Asia Pacific, Johnson & Johnson International, Inc., Co-chair, Asia Pacific American Pharma Congress, Singapore
|

Clivetty Martinez, PhD
Regional Vice President Latin America, Office of Healthcare Compliance and Privacy, Johnson & Johnson International, Inc., Chair, Latin American Ethics and Compliance Network, Co-chair, Latin American Pharma Congress, Miami, FL |

Vincenzo Salvatore, Esq.
Senior Counsel, Sidley Austin LLP, Professor of International Law, University of Insubria, Former Head of Legal Service, European Medicines Agency, Varese, Italy |

Yuet-Ming Tham, Esq.
Partner, Sidley Austin LLP, Hong Kong
|

Roeland Van Aelst
Vice President EMEA & Canada, Office of Health Care Compliance & Privacy, Johnson & Johnson International, Inc., Board Member, International Society of Healthcare, Ethics and Compliance Professionals (ethics), Co-chair, Latin American Pharma Congress, Brussels, Belgium
|

Michael K. Volz, LLM
Group Compliance Officer, Merck KgaA, Frankfurt Am Main, Germany |

Brian Riewerts
Partner, Global Pharmaceuticals and Life Sciences, PwC, Baltimore, MD (Moderator)
|
|
|
|
|

|
EARLY BIRD REGISTRATION
- SAVE $200 -
|
Register by Friday, October 5, 2012 for Early Bird discount and save up to $200.
Click here to register.
|
|
|
|
REGISTER FOR PCF INDIVIDUAL AND GROUP REGISTRATION DISCOUNTS
|
|
Click here to register.
|
|
|
|
BROCHURE NOW AVAILABLE
|
|
Click here to download the brochure.
|
|
|
|
|
SPONSORED BY:
|

The Pharmaceutical Compliance Forum (PCF) is a coalition of senior compliance professionals and legal counsel from more than 50 of the largest research-based pharmaceutical manufacturers. The PCF was founded in early-1999 by compliance professionals from the pharmaceutical industry to promote effective corporate compliance programs. The members meet twice a year, for two days, focusing on open and informal sharing of compliance information, best practices, and current developments in the field, and sponsors a two-day international compliance congress in the Spring and a three-day US compliance congress each Fall.
|
|
|
|
VOTE BY ABSENTEE BALLOT
|
|
Click here for information regarding voting by absentee ballot in all 50 states.
|
|
|
|
CO CHAIRS
|

Gary DelVecchio
Executive Director, US Pharmaceutical Compliance and Ethics, Bristol-Myers Squibb Company, Plainsboro, NJ
|

Margaret K. Feltz, Esq.
Director, Corporate Compliance, Purdue Pharma LP, Stamford, CT
|

Kelly B. Freeman, PhD
Ethics and Compliance Officer, Eli Lilly and Company, Indianapolis, IN |

Michael L. Shaw
Vice President & Compliance Officer, GlaxoSmithKline-NA Pharmaceuticals, Former Senior Counsel, Office of Inspector General, Philadelphia, PA
|
|
|
BEST PRACTICES POSTER BOARD RECEPTION CHAIR
|

Danielle Bacco
Manager Corporate Compliance, Purdue Pharma LP, Stamford, CT
|
|
|
|
|
GRANTORS:
|
|
DIAMOND
|


|
|
SILVER
|




|
|
BRONZE
|













|
|
MEDIA PARTNERS:
|





|
|
FREE TRIAL OFFER FOR INSIDE HEALTH POLICY
|
 Inside Washington Publishers is offering friends of the Thirteenth Pharma Congress a free four-week trial subscription to their newsletter Inside Health Policy. The free trial includes all regular subscriber services, including an e-mail alert every business day as a reminder of what's been added to the site.
Inside Washington Publishers is offering friends of the Thirteenth Pharma Congress a free four-week trial subscription to their newsletter Inside Health Policy. The free trial includes all regular subscriber services, including an e-mail alert every business day as a reminder of what's been added to the site.
|
|
2012 PCF PHARMA CONGRESS PLANNING COMMITTEE
|
Gary DelVecchio
Executive Director, US Pharmaceutical Compliance and Ethics, Bristol-Myers Squibb Company (Co chair)
Margaret K. Feltz
Associate Director, Corporate Compliance, Purdue Pharma LP (Co chair)
Kelly B. Freeman, PhD
Director, US Affiliate, Compliance and Ethics, Eli Lilly and Company (Co chair)
Michael L. Shaw, Esq.
Vice President & Compliance Officer, GlaxoSmithKline-NA Pharmaceuticals (Co chair)
Ted Acosta, Esq.
Principal, Ernst & Young LLP
Timothy Ayers, MPH, JD
Vice President and Chief Compliance Officer, Dendreon
Wayne Baker
Senior Vice President and Chief Sales Officer, Advanced Health Media LLC
Scott Bass, Esq.
Partner, Sidley Austin LLP
John T. Bentivoglio, Esq.
Partner, Skadden Arps LLP
Eve M. Brunts, Esq.
Partner, Ropes & Gray
William E. Buzzeo, MS
Vice President and General Manager Compliance Solutions Division, Cegedim Relationship Management
Sujata T. Dayal
Corporate Vice President and Chief Compliance Officer, Global Operations, Biomet, Inc.
Fred Eaton, MBA
Partner, Polaris Management Partners
Jeffrey E. Fleming, Esq.
Americas & US Compliance Officer, AstraZeneca Pharmaceuticals LP
Thomas Forrester, Esq.
Vice President, US Legal Affairs and General Counsel, Lundbeck Inc.
Gary F. Giampetruzzi, Esq.
Vice President and Assistant General Counsel, Head of Government Investigations, Pfizer Inc.
Wendy C. Goldstein, Esq.
Partner, Epstein Becker & Green
Alessandra N. Hawthorne
Vice President, Chief Ethics and Compliance Officer, Boehringer Ingelheim USA, Inc.
Michael Hercz, Esq.
Director, Audit and Enterprise Risk Services, Deloitte & Touche LLP
Elizabeth V. Jobes, Esq.
Senior Vice President, Chief Compliance Officer, Auxilium Pharmaceuticals Inc.
Jonathan Kellerman
Partner, Global Pharmaceutical Advisory Services Group, PwC LLP
Daniel Kracov, Esq.
Partner, Arnold & Porter
Maxine Nogard
Senior Director, Global Corporate Compliance, Biogen Idec, Inc.
John Patrick Oroho, Esq.
Executive Vice President and Chief Strategy Officer, Porzio Pharmaceutical Services, LLC; Principal, Porzio, Bromberg & Newman
Lawrence P. Platkin
Vice President and Compliance Officer, Bayer Healthcare LLC
Arjun Rajaratnam
Chief Compliance Officer, Smith & Nephew
Kelly N. "Nikki" Reeves, MPA, JD
Partner, King & Spalding LLP
Susan Romanus
Vice President, Chief Ethics & Compliance Officer, Daiichi Sankyo
Jeff Rosenbaum
Vice President, Chief Compliance Officer, Vertex Pharmaceuticals
Karen Patruno Sheehy, Esq.
Vice President, US Corporate Compliance Officer, Sanofi
Eric Siegel, Esq.
Executive Vice President & General Counsel, Incyte Corporation
Paul J. Silver
Managing Director, Huron Consulting Group
Jack T. Tanselle
Director, Navigant Consulting, Inc.
Caroline West, Esq.
Senior Vice President, Chief Compliance and Risk Officer, Shire Pharmaceuticals, Inc.
Ronald L. Wisor, Jr., Esq.
Partner, Hogan Lovells US LLP
Christopher D. Zalesky
Executive Director, World Wide Office of Health Care Compliance & Privacy, Johnson & Johnson
Richard L. Zimmerer
Partner, Forensic Advisory Services, KPMG LLP
|
|
2011 PHARMA CONGRESS CONTENT NOW AVAILABLE
|
The 2011 Pharma Congress conference content is now available in a variety of formats.
Click here for more info.
|
|
FEATURING VIDEO PRESENTATIONS BY GLOBAL PHARMA CEOs:
|
|
2012 Sixth International Pharma Congress Keynote Address by
|

Christopher A. Viehbacher
Chief Executive Officer, Sanofi, Chairman, Genzyme, Paris, France
|
|
2011 Fifth International Pharma Congress Keynote Address by
|

David Brennan
Former Chief Executive Officer, AstraZeneca, President, IFPMA, Past Chairman, PhRMA, London, UK
|
|
2012 Twelfth Pharmaceutical Regulatory Compliance Congress and Best Practices Forum by
|
John C. Lechleiter, PhD
Chairman, President and Chief Executive Officer, Eli Lilly and Company, Chairman-elect, PhRMA, Indianapolis, IN
|
|
PAST GLOBAL PHARMA CONGRESS CITIES
|

Washington, DC

Brussels

Paris

Rome

Berlin

Istanbul

Singapore

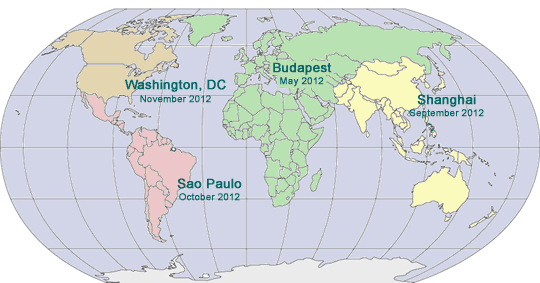
2012 - Budapest, Hungary

2012 - Shanghai, China

2012 - São Paulo, Brazil
|
|

 This site complies with the HONcode standard for trustworthy health information: This site complies with the HONcode standard for trustworthy health information:
verify here.


|
|
|
SAVE THE DATE
|
|
SIXTH INTERNATIONAL PHARMACEUTICAL REGULATORY AND COMPLIANCE CONGRESS
|
A Hybrid Conference and Internet Event
Sponsored by the Pharmaceutical Compliance Forum
May 14 - 16, 2012
Budapest, Hungary
www.InternationalPharmaCongress.com
|
|
SECOND ASIA PACIFIC
PHARMACEUTICAL COMPLIANCE CONGRESS
|
A Hybrid Conference and Internet Event
Sponsored by Pharmaceutical Compliance Forum and Asia Pacific Healthcare Industry Compliance Team
September 11 - 13, 2012
Intercontinental Shanghai Pudong Hotel
Shanghai, China
www.AsianPharmaCongress.com
|
|
FIRST LATIN AMERICAN PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
|
A Hybrid Conference and Internet Event
Sponsored by Pharmaceutical Compliance Forum and Latin American Ethics and Compliance Network
October 2 - 4, 2012
Hilton Sao Paolo Morumba
Sao Paulo, Brazil
www.LatinAmericanPharmaCongress.com
|
|
|



