|
2013 Seventh International Pharma Compliance Congress, May 21 - 23, 2013, Madrid, Spain, Keynoted by Huguette Labelle, CC, PhD, LLD, Chair, Transparency International; Early Bird Registration Rate Ends this Friday, April 19, 2013
|
- A Hybrid Conference and Internet Event
- Sponsored by International Society of Healthcare Compliance Professionals
- Co-sponsored by the Pharmaceutical Compliance Forum
- Held May 21 - 23, 2013
- Onsite at the Meliá Castilla Hotel, Madrid, Spain
- In Your Own Office or Home live via the Internet with 24/7 Access for Six Months
- www.InternationalPharmaCongress.com
PRESS RELEASE
Phone: 800-503-8171
Email: registration@hcconferences.com
Website: www.InternationalPharmaCongress.com |
|
FEATURING KEYNOTE ADDRESS BY
|

Huguette Labelle, CC, PhD, LLD
Chair, Transparency International, Companion of the Order of Canada, Former Deputy, Canadian Secretary of State, Transport Canada, Public Service Commission and Canadian International Development Agency, Berlin, Germany
|
|
|
WASHINGTON DC USA -- PHARMA UPDATE NEWS SERVICE™ -- APRIL 17, 2013: The Seventh International Pharmaceutical Compliance Congress, www.InternationalPharmaCongress.com, will take place on May 21 - 23, 2013 at the Meliá Castilla Hotel in Madrid, Spain. Registrants may attend the event in person or online (live webcast and archived for 6 months).
GABOR DANIELFY MEMORIAL
KEYNOTE ADDRESS
|

Elvira Sanz
President, Pfizer Spain, President, Farmaindustria, Madrid, Spain
|
|
|
ETHICS
KEYNOTE ADDRESS
|

Marc Le Menestrel, PhD
Visiting Professor of Ethics, INSEAD, Associate Professor, University
Pompeu Fabra (Barcelona), Barcelona, Spain
|
|
|
TRANSFORMING THE COMPLIANCE PROFESSION KEYNOTE ADDRESS
|

Arthur Muratyan, Esq.
Secretary General and Board Member, International Society of Healthcare, Ethics and Compliance Professionals (ethics), Former Vice President-Head of Legal Corporate, and Global Compliance Officer, Sanofi, Paris, France
|
|
|
TRANSPARENCY
INT'L KEYNOTE ADDRESS
|

Huguette Labelle, CC, PhD, LLD
Chair, Transparency International, Companion of the Order of Canada, Berlin, Germany
|
|
|
OTHER KEYNOTE SPEAKERS
|

Reinhard Angelmar, PhD
Salmon and Rameau Fellow of Healthcare, Management Professor of Marketing, INSEAD, Appointments, Sloan School (MIT), Stockholm School of Economics, ESSEC Business School, and Universite Paris Dauphine, Fontainebleau, France |

Carl H. Coleman, JD
Professor of Law and Director of Global Initiatives, Center for Health & Pharmaceutical Law & Policy and Gibbons, Institute of Law, Science & Technology, Seton Hall University School of Law, Former Bioethics and Law Adviser, World Health Organization (WHO), Newark, NJ |

Jose F. Zamarriego Izquierdo
Director Unidad de Supervision Deontologica, FARMAINDUSTRIA, Madrid, Spain
|

Tamara Music
Manager, Influenza Vaccines & Code Compliance, IFPMA, Geneva, Switzerland |

Marie-Claire Pickaert
Deputy Director General, European Federation of Pharmaceutical Industries and Associations (EFPIA), Brussels, Belgium
|

Heather Simmonds
Director and Chair, Code of Practice Panel, Prescription Medicines Code of Practice Authority, London, UK |

Andy Powrie-Smith
Director for Trust and Reputation, Association of the British Pharmaceutical Industry (ABPI), London, UK
|
|
|
|
A SPECIAL COMPLIMENTARY, INVITATION-ONLY
SPANISH COMPLIANCE ROUNDTABLE
On Tuesday, May 21, 2013 immediately prior to the Congress
Sponsored by FARMAINDUSTRIA FEATURING
|

Arancha Burgos
Spanish Code Surveillance Unit, FARMAINDUSTRIA, Madrid, Spain |

Santiago Páramo
Spanish Code Surveillance Unit, FARMAINDUSTRIA, Madrid, Spain |

Olga Sanz
Spanish Code Surveillance Unit, FARMAINDUSTRIA, Madrid, Spain
|

Julián Zabala
Head of Communications, FARMAINDUSTRIA, Madrid, Spain |

Jose F. Zamarriego Izquierdo
Director Unidad de Supervision Deontologica, FARMAINDUSTRIA, Madrid, Spain (Moderator)
|
|
|
|
|
|
PLENARY SESSIONS
|
- Business Ethics for Pharma and Device Companies
- Roundtable on Integrating Ethics Theory into Ethics Practice
- Global Anticorruption Roundtable
- EU Transparency Roundtable
- Compliance Issues and Congresses
- The Role of the International Society of Healthcare Ethics and Compliance Professionals (ethics)
- Compliance as a Profession: Common Body of Knowledge, Training, Professional Ethics and Professional Certification
|
|
INTERNATIONAL PHARMA CONGRESS IS
|

|
|
|
|
AND MINI SUMMITS
|
- Mini Summit I: Basic Compliance Training
- Mini Summit II: Global Auditing and Monitoring Best Practices I
- Mini Summit III: Annual MEA Compliance Roundtable
- Mini Summit IV: Social Media, Apps and eDigital Space
- Mini Summit V: Crucial Compliance Resources And Non-Material Support For Compliance Officers
- Mini Summit VI: INSEAD Case Study
- Mini Summit VII: Annual CEE Compliance Update
- Mini Summit VIII: Advanced Issues In Global Transparency
- Mini Summit IX: Global Compliance Issues And Initiatives In The Medical Device Sector
- Mini Summit X: Global Anti-Bribery Compliance
- Mini Summit XI: Global Auditing and Monitoring Best Practices II
- Mini Summit XII: Global Compliance Issues Update
- Mini Summit XIII: Public Procurement and Pharma Pricing Update
- Mini Summit XIV: Global Compliance Codes Update
- Mini Summit XV: Pre- And Post-Transaction Due Diligence For Compliance
- Mini Summit XVI: Making Use Of Case Studies As An Educational Tool
- Mini Summit XVII: Achilles Heel in Managing Global Corruption and Preventing Your Initiative's Downfall
|
|
|
|
ETHICS UPDATE
|

Michael Hercz, Esq.
Director, Audit and Enterprise Risk Services, Deloitte & Touche LLP; Former Vice President and Chief Compliance Officer, Victory Pharmaceuticals, Inc., Costa Mesa, CA, USA |

Dan Ostergaard
Founder and Chief Executive Officer, Integrity by Design, Former Head of Corporate Integrity and Compliance, Novartis AG, Basel, Switzerland |

Guido Palazzo, PhD
Professor of Business Ethics, University of Lausanne, Visiting Fellow, Universities of Nottingham and Oxford, Member, Editorial Boards, Business Ethics Quarterly, and Business & Society, Lausanne, Switzerland
|

Andy Powrie-Smith
Director for Trust and Reputation, Association of the British Pharmaceutical Industry (ABPI), London, UK |

Lori Queisser
Principal, Advisory Services, KPMG LLP, Former Senior Vice President, Global Compliance and Business Practices, Schering-Plough Corporation, Former Member, PCF Executive Committee, Indianapolis, IN, USA |

Paul B. Woods, BPharm, MA, MRPharmS
Independent Consultant; Former Global Compliance Policy Director, AstraZeneca, Macclesfield, Cheshire, UK
|
|
|
|
|
GLOBAL ANTICORRUPTION UPDATE
|

Peter Dieners, Esq.
Partner, Clifford Chance, Düsseldorf, Germany |

Gerry Fujimoto, CPA
Partner, Forensic & Dispute Services, Deloitte Financial Advisory Services, LLP, San Francisco, CA |

Gary F. Giampetruzzi, Esq.
Vice President and Assistant General Counsel, Head of Government Investigations, Pfizer Inc., New York, NY, USA
|

Edward F. Hanover, Esq.
General Counsel, Novo Nordisk Region International Operations A/S, Zurich, Switzerland |

Marc L. Miller
Partner, KPMG LLP, New York, NY, USA |

Lewis Morris, Esq.
Senior Counsel, Adelman, Sheff and Smith LLC, Former Chief Counsel to the Inspector General, Office of Inspector General, US Department of Health and Human Services, Annapolis, MD, USA
|

Edward A. Rial, JD
Partner and Global Leader, FCPA Consulting Services, Deloitte Financial Advisory Services LLP; Former Assistant US Attorney, US Attorney's Office, Eastern District of New York, New York, NY |

Vivian Robinson, Esq.
Partner, McGuireWoods; Former General Counsel of the UK Serious Fraud Office; Former Head, QEB Hollis Whiteman Chambers; Recorder of the Crown Court and Treasurer of Inner Temple, London, UK |

Joseph B. Tompkins, Jr.
Partner, Sidley Austin LLP, Former Deputy Chief of the Fraud Section, Criminal Division, United States Department of Justice, Washington, DC, USA
|

Juan Valderas
Partner, Forensic & Dispute Services, Deloitte Financial Advisory Services, LLP, Madrid, Spain |

Ted Acosta, Esq.
Principal, Ernst & Young LLP, Former Senior Counsel, Office of Inspector General, US Department of Health and Human Services, New York, NY, USA and Paris, France (Moderator)
|
|
|
|
|
GLOBAL TRANSPARENCY UPDATE
|

Dr. Michael Bartke
Director Compliance Management, DAIICHI SANKYO EUROPE GmbH, Co-chair, EFPIA Compliance Workgroup, Munich, Germany |

Andy Bender, MS, MBA
President, Polaris, New York, NY, USA |

Carsten Blaesberg
Chief Consultant, Lif Denmark, The Danish Association of the Pharmaceutical Industry, Copenhagen, Denmark
|

Julie Bonhomme, Esq.
Legal Manager, Legal and Compliance Department, LEEM, Paris, France |

William E. Buzzeo, MS
Vice President and General Manager, Global Compliance Solutions, Cegedim Relationship Management, Richmond, VA, USA |

Daniel A. Kracov, Esq.
Partner and Head, FDA and Healthcare Practice, Arnold & Porter, Washington, DC, USA
|

Evelyne Lemaire
Director, Global Pharmaceutical and Life Sciences, PwC LLP, Zurich, Switzerland |

Andrew Parks
Director, KPMG, McLean, VA, USA
|

Heather Simmonds
Director, Prescription Medicines Code of Practice Authority, London, UK |

Matthijs M. van Blokland, Esq.
General Counsel, Prosensa, General Counsel and Senior Policy Advisor Legal Affairs, Association Innovative Medicines, Nefarma, Board Member, Stichting CGRz, Amsterdam, The Netherlands
|
|
|
|
|
GLOBAL COMPLIANCE ISSUES AND CODES UPDATES
|

John Auerbach, MA
Partner, Fraud Investigation and Dispute Services, Ernst & Young, Shanghai, China |

Bulent Becan
Managing Partner, Atuva Management Consultancy, and Trade Ltd.; Consultant in Ethics Issues, Turkish Research Based, Pharmaceutical Companies Association (AIFD), Istanbul, Turkey |

Joe Henein
President and Chief Executive Officer, NewBridge Pharmaceuticals, Former Regional Managing Director, Middle East, and North Africa, Wyeth Pharmaceuticals, Dubai, United Arab Emirates
|

Vivian Lima-Barnett
OEC Director, Latin America, Abbvie, Co-chair, Latin American Pharma Congress, Abbott Park, IL, USA |

Juan Francisco Millán Soberanes, MD
Secretary and Executive Director, CETIFARMA, Mexico City, Mexico |

Keith M. Korenchuk, JD, MPH
Partner, Arnold & Porter LLP, Washington, DC, USA
|
|
|
|
|
GLOBAL COMPLIANCE ISSUES IN THE MEDICAL DEVICE SECTOR
|

Suzanne Durdevic, DEA, LLM
Senior Director, General Counsel, EMEA, Boston Scientific International; Chair, Legal Affairs Group, EUCOMED, Paris, France |

Anthony McQuillan, Esq.
Vice President Legal and Compliance EMEA, Medtronic; Chair, EUCOMED Code Committee, Tolochenaz, Switzerland |

Elisabethann Wright, Esq.
Partner, Hogan Lovells; Former Senior Legal Officer and Hearing Officer, EFTA Surveillance Authority, Brussels, Belgium
|

Brian Riewerts
Partner, Global Pharmaceuticals and Life Sciences, PwC LLP Baltimore, MD, USA (Co-moderator) |

Roeland Van Aelst
Vice President EMEA & Canada, Health Care Compliance and Privacy, Johnson & Johnson; Board Member and Co-chair, Strategic Committee, International Society of Healthcare Ethics and Compliance Professionals (ethics), Brussels, Belgium (Co-moderator)
|
|
|
|
|
CONGRESSES AND REALITIES: CROSS-BORDER CONFLUENCE, TERRITORIAL ISSUES VS. GLOBAL ACTIVITIES, MIXING IFPMA/EFPIA CODES WITH CONGRESS AND SOCIETY RULES
|

Marie-Claire Pickaert
Deputy Director General, European Federation of Pharmaceutical Industries and Associations (EFPIA), Brussels, Belgium |

Jeff Rosenbaum
Vice President, Chief Compliance Officer, Vertex Pharmaceuticals, Former Executive Director, Global Head of Ethics & Compliance Novartis Oncology, Boston, MA |

Debra C. Forster, Esq.
Global Compliance Officer, Novartis Pharma AG, Basel, Switzerland
|

Christian-Claus Roth
Head of Congresses and Conventions, Novartis Pharma; Co-President, International Pharmaceutical Congress Advisory Association (IPCAA), Basel, Switzerland |

Ann Beasley Bacon
Vice President Corporate Compliance, International, Biogen Idec International, GmbH (as of March 1); Former Global Compliance Officer, Novartis Pharma AG; Board Member and Co-chair, Strategic Committee, International Society of Healthcare, Ethics and Compliance Professionals (ethics), Zug, Switzerland (Moderator)
|
|
|
|
|
BASIC COMPLIANCE TRAINING
|

Sue Egan
Director and Principal Consultant, Sue Egan Associates, Editor, Life Science Compliance, Former Vice President Compliance, AstraZeneca, Great Missenden, Buckinghamshire, UK
|
|
|
|
CRUCIAL COMPLIANCE RESOURCES
|

Tomasz Kruk, LL M, MBA
Director Global Ethics and Compliance, Actavis, Zug, Switzerland
|
|
|
EMERGING COMPLIANCE ISSUES: SOCIAL MEDIA, APPS AND eDIGITAL SPACE
|

Bulent Becan
Managing Partner, Atuva Management Consultancy and Trade Ltd., Consultant in Ethics Issues, Turkish Research Based Pharmaceutical Companies Association (AIFD), Istanbul, Turkey |

Noelia de Miguel, PhD, Esq.
Senior Associate and Head of Life, Sciences Regulatory Spain, Hogan Lovells International LLP, Madrid, Spain
|

Denise Silber
President, Basil Strategies Digital Consultancy and Organizers, Doctors 2.0 & You Conference, Paris, France |

Philippe Cloarec
European Ethics and Compliance Associate - CCEP-I, Eli Lilly and Company, Geneva, Switzerland (Moderator)
|
|
|
|
|
BEST PRACTICES IN GLOBAL AUDITING AND COMPLIANCE MANAGEMENT SYSTEMS
|

Yogesh Bahl, MBA
Partner, National Practice Leader - Life Sciences, Deloitte Financial Advisory Services LLP, New York, NY, USA |

Andy Bender, MS, MBA
President, Polarism New York, NY, USA |

L. Stephan Vincze, JD, LLM, MBA
Director, Audit and Enterprise, Risk Services, Deloitte & Touche LLP; Former Vice President, Ethics & Compliance Officer and Privacy Officer, TAP Pharmaceutical Products Inc., Boston, MA, USA (Moderator)
|
|
|
|
|
THIRD PARTY AUDITS
|
George Fife
Director, EMEA Compliance and Ethics, Bristol-Myers, Squibb, Paris, France |

Michele Tagliaferri, Esq.
Counsel, Sidley Austin LLP, Brussels, Belgium |

Melda Tanyeri, MS, MBA
Senior Manager, Fraud Investigation and Dispute Services, Ernst & Young LLP, Paris, France
|
|
|
|
|
PRE- AND POST-TRANSACTION DUE DILIGENCE
|
Brian E. Kowalski, Esq.
Associate, Latham & Watkins, Washington, DC, USA |

Greg Crouse
Principal, Ernst & Young LLP, Moscow, Russia (Moderator)
|
|
|
|
|
COMPLIANCE CASE STUDIES
|

Nancy A. Grygiel, LD, LLM
Senior Director, International Business Compliance, Allergan, Irvine, CA, USA |

Jonathon Kellerman
Principal, Pharmaceutical and Life, Sciences Advisory Services, PwC LLP, Florham Park, NJ, USA (Co-moderator) |

Gerard Geneen
Vice President, Compliance Officer, Pharma Europe GlaxoSmithKline, Brentford, Middlesex, United Kingdom (Co-moderator)
|
|
|
|
|
CENTRAL AND EASTERN (CEE) UPDATE
|

Oleksiy Bezhevets, JD, MBA
Partner, ARCA Law Firm; Partner, Legal Alliance Company, Ukraine |

Paul J. Melling, Esq.
Founding Partner, Baker & McKenzie - CIS, Limited, Moscow, Russia |

Mariusz Witalis
Partner, Fraud Investigation and Dispute Services, Ernst & Young LLP, Warsaw, Poland (Moderator)
|
|
|
|
|
MIDDLE EAST AFRICA (MEA) UPDATE
|

Hulya Baran
Director, Ethics and Compliance TMEA - CIS, Eli Lilly and Company, Istanbul, Turkey |

Meltem Ozker Gunduz
Legal Affairs and Compliance Director, Business Area Near East, Novo Nordisk, Istanbul, Turkey |

Liz MacGillivray
Compliance Director, Anti-Bribery, Country Support & Monitoring, Group Integrity and Compliance, Novartis International AG, Basil, Switzerland
|

Laura Nassar
Regional Pharma HCC Officer Emerging Markets, Johnson & Johnson, Beirut, Lebanon |

Joe Henein
President and Chief Executive Officer, NewBridge Pharmaceuticals, Former Regional Managing Director, Middle East, and North Africa, Wyeth Pharmaceuticals, Dubai, United Arab Emirates (Moderator)
|
|
|
|
|
COMPLIANCE ISSUES FOR SMALL-MID SIZE PHARMA AND BIOTECH COMPANIES
|

Maurits J.F. Lugard, MA, JD, LLM
Partner, Sidley Austin LLP, Former Member of the European Commission's Legal Service, Brussels, Belgium |

John Patrick Oroho, Esq.
Executive Vice President and Chief Strategy Officer, Porzio Life Sciences, LLC; Principal, Porzio, Bromberg & Newman PC, Morristown, NJ, USA
|
|
|
|
|
EFFECTIVE MANAGEMENT OF CROSS-BORDER EXPERT ENGAGEMENTS
|

Wayne Baker
Senior Vice President and Chief Sales Officer, AHM, New Providence, NJ, USA |

Iain Holloway-McLean
Medical Operations Director, GlaxoSmithKline, Brentford, UK
|
|
|
|
|
SPECIAL SPANISH STAKEHOLDERS ROUNDTABLE
|

Dra. Begoña Barragán
President, Spanish Group of Cancer Patients, Madrid, Spain |

Dr. Antonio Cachá
Deputy Director General for Evaluation and Control, Healthcare Ministry of the Regional Government, Community of Madrid, Madrid, Spain
|

Dra. Neus Rams I Pla
Deputy Director General of Pharmacy and Sanitary Products, Generalitat de Catalunya, Barcelona, Spain |

Dr. Miguel Ángel Caracuel Ruiz
Vice-President, Spanish Federation of Scientific Medical Associations, Científico Médicas Españolas (FACME), Madrid, Spain
|
|
|
|
|
SAVE THE DATE
|
|
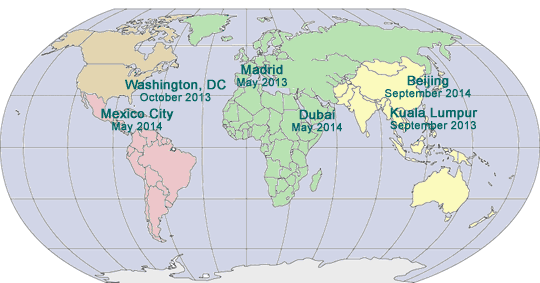
FIFTH ANNUAL SUMMIT ON DISCLOSURE, TRANSPARENCY AND AGGREGATE SPEND
|
A Hybrid Conference and Internet Event
February 19 - 21, 2013
The Ritz-Carlton
Washington, DC
www.DisclosureSummit.com
|
|
SEVENTH INTERNATIONAL PHARMACEUTICAL COMPLIANCE CONGRESS
|
A Hybrid Conference and Internet Event
Sponsored by International Society of Healthcare Compliance Professionals
Cosponsored by Pharmaceutical Compliance Forum
May 21 - 23, 2013
Meliá Castilla Hotel
Madrid, Spain
www.InternationalPharmaCongress.com
|
|
THIRD ASIA PACIFIC
PHARMACEUTICAL COMPLIANCE
CONGRESS
|
A Hybrid Conference and Internet Event
Sponsored by Asia Pacific Healthcare Industry Compliance Team
Cosponsored by Pharmaceutical Compliance Forum
September 10 - 12, 2013
Kuala Lumpur, Malaysia
www.AsianPharmaCongress.com
|
|
FOURTEENTH PHARMACEUTICAL REGULATORY AND COMPLIANCE CONGRESS
|
A Hybrid Conference and Internet Event
Sponsored by Pharmaceutical Compliance Forum
October 28 - 30, 2013
Hyatt Regency on Capitol Hill
Washington, DC
www.PharmaCongress.com
|
|
|
|
|
EARLY BIRD REGISTRATION
- SAVE UP TO €200 -
|
Register by Friday, April 19, 2013 for Early Bird Discount and save up to €200.
Click here to register.
|
|
|
|
BROCHURE NOW AVAILABLE
|
Click here to view the brochure.
|
|
|
|
|
SPONSORED BY:
|
|

|
|
CO-SPONSORED BY:
|
|

|
|
MEDIA PARTNERS
|



|
|
CONGRESS CO CHAIRS
|

Ann Beasley Bacon
Vice President Corporate Compliance, International, Biogen Idec International, GmbH (as of March 1); Former Global Compliance Officer, Novartis Pharma AG; Board Member and Co-chair, Strategic Committee, International Society of Healthcare, Ethics and Compliance Professionals (ethics), Zug, Switzerland
|

Dominique Laymand, Esq.
Vice President Compliance & Ethics EMEA, (Europe, Middle-East, Africa, Russia and Turkey), Bristol-Myers Squibb, President, International Society of Healthcare, Ethics and Compliance Professionals (ethics), Paris, France
|

Roeland Van Aelst
Vice President EMEA & Canada, Johnson & Johnson, Board Member and Co-chair, Strategic Committee, International Society of Healthcare, Ethics and Compliance Professionals (ethics), Brussels, Belgium
|
|
|
|
GRANTORS:
|
|
DIAMOND
|

|
|
PLATINUM
|

|
|
GOLD
|


|
|
SILVER
|

|
|
BRONZE
|







|
|
INTERNATIONAL PHARMA CONGRESS PLANNING COMMITTEE
|
Ann Beasley Bacon
Vice President Corporate Compliance, International, Biogen Idec International, GmbH (as of March 1); Zug, Switzerland (Co chair)
Dominique Laymand, Esq.
Vice President Compliance & Ethics EMEA (Europe, Middle-East, Africa, Russia and Turkey), Bristol-Myers Squibb, Paris, France (Co chair)
Michael J. Morgan
Ethics and Compliance Officer, Australia, Canada and Europe, Eli Lilly and Company, Windlesham, Surrey, United Kingdom (Co chair)
Roeland Van Aelst
Vice President EMEA & Canada, J&J Office Health Care Compliance & Privacy, Johnson&Johnson, Brussels, Belgium (Co chair)
Ted Acosta, Esq.
Principal, Ernst & Young LLP, Former Senior Counsel, Office of Inspector General, US Department of Health and Human Services, New York, NY, USA and Paris, France
Ronny Arijs
Vice President Global Sustainability & Chief Compliance Officer, Grunenthal, Former Senior Compliance Manager GE Healthcare, Brussels, Belgium
Yogesh Bahl, MBA
Partner, National Practice Leader - Life Sciences, Deloitte Financial Advisory Services LLP, New York, NY, USA
Wayne Baker
Senior Vice President & Chief Sales Officer, Advanced Health Media LLC, New Providence, NJ, USA
Bulent Becan
Managing Partner, Atuva Management Consultancy and Trade Ltd., Consultant in Ethics Issues, Turkish Research Based Pharmaceutical Companies Association (AIFD), Istanbul, Turkey
Andy Bender, MS, MBA
President, Polaris, New York, NY, USA
John T. Bentivoglio, Esq.
Partner, Skadden Arps LLP, Former Special Counsel for Healthcare Fraud, and Chief Privacy Officer, US Department of Justice, Washington, DC, USA
Hugh C. Bigwood
Ethics and Compliance Officer, International Operations, Abbott Laboratories, Maidenhead, Berkshire, United Kingdom
William E. Buzzeo, MS
Vice President and General Manager, Global Compliance Solutions, Cegedim Relationship Management, Richmond, VA, USA
Antonio Cavallaro
Compliance & Internal Auditing Senior Manager, Takeda Italia, Rome, Italy
Peter Dieners, Esq.
Partner, Clifford Chance, Düsseldorf, Germany
Pierre E. Dupourque
Regional Compliance Director, Corporate Compliance, International Investigations and Programs, Pfizer Inc., Mannheim, Germany
Sue Egan
Director and Principal Consultant, Sue Egan Associates, Editor, Life Science Compliance, Former Vice President Compliance, AstraZeneca, Great Missenden, Buckinghamshire, UK
Annette Schutt Fiig
Director, Risk Office, Novo Nordisk, Copenhagen, Denmark
Gerard Geneen
Vice President, Compliance Officer, Pharma Europe, GlaxoSmithKline, Brentford, Middlesex, United Kingdom
Nancy A. Grygiel, JD, LLM
Senior Director, Corporate Compliance, Business Compliance, International, Allergan, Irvine, CA, USA
Jose F. Zamarriego Izquierdo
Director Unidad de Supervision Deontologica, FARMAINDUSTRIA, Madrid, Spain
Jonathon Kellerman
Principal, Pharmaceutical and Life Sciences Advisory Services, PwC LLP, Florham Park, NJ, USA
Keith M. Korenchuk, JD, MPH
Partner, Arnold & Porter LLP, Washington, DC, USA
Tomasz Kruk
Director Global Ethics & Compliance, Actavis, Zug, Switzerland
Paul J. Melling, Esq.
Founding Partner, Baker & McKenzie - CIS, Limited, Moscow, Russia
Marc L. Miller
Partner, KPMG LLP, New York, NY, USA
Maxine Nogard
Senior Director, Global Corporate Compliance, Biogen Idec Inc., Weston, MA, USA
John Patrick Oroho, Esq.
Executive Vice President and Chief Strategy Officer, Porzio Life Sciences, LLC; Principal, Porzio, Bromberg & Newman PC, Morristown, NJ, USA
Eric Siegel
Executive Vice President & General Counsel, Incyte Corporation, Wilmington, DE, USA
Joseph B. Tompkins, Jr.
Partner, Sidley Austin LLP, Former Deputy Chief of the Fraud Section, Criminal Division of the United States Department of Justice, Washington, DC, USA
Paul B. Woods, BPharm, MA, MRPharmS
Independent Consultant, Former Global Compliance Policy Director, AstraZeneca, Macclesfield, Cheshire, UK
Elisabethann Wright, Esq.
Partner, Hogan Lovells, Former, Senior Legal Officer and Hearing Officer, EFTA Surveillance Authority, Brussels, Belgium
|
|
|
INTERNATIONAL PHARMA CONGRESSES
|

2007 - Brussels, Belgium

2008 - Paris, France

2009 - Rome, Italy

2010 - Berlin, Germany

2011 - Istanbul, Turkey

2011 - Singapore

2012 - Budapest, Hungary

2012 - Shanghai, China

2012 - São Paulo, Brazil

2013 - Madrid, Spain

2013 - Kuala Lumpur, Malaysia
|
|
|
FEATURING VIDEO PRESENTATIONS BY PHARMA LEADERS:
|
|
2012 Second Asia Pacific Pharma Congress Keynote Address by
|

Mark Mallon, MBA
Regional Vice President Asia Pacific and President China, AstraZeneca, Former Executive Vice-President and Chief Operating Officer, AstraZeneca KK (Japan), Shanghai, China
|
|
2012 Sixth International Pharma Congress Keynote Address by
|

Christopher A. Viehbacher
Chief Executive Officer, Sanofi, Chairman, Genzyme, Paris, France
|
|
2011 Twelfth Pharmaceutical Regulatory Compliance Congress and Best Practices Forum by
|
John C. Lechleiter, PhD
Chairman, President and Chief Executive Officer, Eli Lilly and Company, Chairman-elect, PhRMA, Indianapolis, IN
|
|
2011 Fifth International Pharma Congress Keynote Address by
|

David Brennan
Former Chief Executive Officer, AstraZeneca, President, IFPMA, Past Chairman, PhRMA, London, UK
|
|
|
|
|