|
|
|
Press Release: The Pharmaceutical Compliance Forum's Eighteenth Pharmaceutical and Medical Device Compliance Congress Features Annual AUSA Roundtable
|
- Sponsored by the Pharmaceutical Compliance Forum
- November 6 - 8, 2017
- Onsite at the Mandarin Oriental, Washington, DC
- Online in Your Own Office or Home Live via the Internet with 24/7 Access for Six Months
- www.PharmaCongress.com
PRESS RELEASE
Phone: 800-503-7419
Email: registration@hcconferences.com
Website: www.PharmaCongress.com |
|
SPEAKER PRESENTATION PROPOSALS
|
Speaker/Presentation Proposals for the Eighteenth Pharma Congress May Be Submitted Through Our Online Form.
- Click Here -
|
|
|
FEATURING ANNUAL AUSA ROUNDTABLE
|

Jacob Elberg, JD
Chief, Health Care and Government Fraud Unit, US Attorney's Office, District of New Jersey, US Department of Justice, Newark, NJ |

Jason Mehta, JD
Assistant US Attorney, United States Attorney's Office Middle District of Florida, US Department of Justice, Jacksonville, FL
|

Gregg Shapiro, JD
Assistant US Attorney, US Attorney's Office, District of Massachusetts, US Department of Justice, Boston, MA
|

John T. Bentivoglio, Esq.
Partner, Skadden Arps LLP, Former Special Counsel for Healthcare Fraud and Chief Privacy Officer, US Department of Justice, Washington, DC (Moderator)
|
|
|
WASHINGTON, DC USA -- PHARMA UPDATE NEWS SERVICE™ -- JUNE 15, 2017: The Pharmaceutical Compliance Forum's Eighteenth Pharmaceutical and Medical Device Compliance Congress, www.PharmaCongress.com, will be held November 6 - 8, 2017 at the Mandarin Oriental in Washington, DC. Registrants may attend the event in person or online (live webcast and archived for 6 months).
|
|
KEYNOTE SPEAKERS
|

Thomas W. Abrams, RPh, MBA
Director, Office of Prescription Drug Promotion, US Food and Drug Administration, Silver Spring, MD |

Mary E. Riordan, Esq.
Senior Counsel, Office of Counsel to the Inspector General, Office of Inspector General, US Department of Health and Human Services, Washington, DC
|
|
|
CO CHAIRS
|

Timothy Ayers, JD, MPH
Vice President, Chief Compliance Officer, Horizon Pharma plc, Chicago, IL |

Matthew D'Ambrosio, JD, MBA
Senior Vice President, Chief Compliance and Ethics Officer, Sunovion Pharmaceuticals Inc., Chair, Pharmaceutical Compliance Forum, Marlborough, MA |
Sujata T. Dayal, JD
Vice President, Health Care Compliance & Privacy, Pharmaceuticals Group, Johnson & Johnson, Titusville, NJ
|

Jeffrey Kawalek, MBA
Senior Director, Ethics & Compliance, North America, Ipsen Biopharmaceuticals, Inc., New York, NY |

Jennifer McGee, JD
Vice President and Chief Compliance Officer, Otsuka America Pharmaceutical, Inc., Rockville, MD |

Margaret Sparks, JD
Associate Vice President, North America Ethics and Business Integrity, Sanofi, US, Bridgewater, NJ
|
|
|
FEATURED FACULTY
|

Thomas W. Beimers, Esq.
Partner, Hogan Lovells, Former Senior Counsel for Administrative and Civil Remedies, Office of the Inspector General, Department of Health and Human Services, Minneapolis, MN |

Andy Bender, MS, MBA
President and Founder, Polaris, New York, NY |

John T. Bentivoglio, Esq.
Partner, Skadden Arps LLP, Former Special Counsel for Healthcare Fraud, and Chief Privacy Officer, US Department of Justice, Washington, DC
|

Susan Dentzer
President and Chief Executive Officer, NEHI (The Network for Excellence in Health Innovation), Analyst on Health Policy, The NewsHour, Washington, DC |

Michelle Drozd, ScM
Deputy Vice President, Policy and Research, Pharmaceutical Research & Manufacturers of America (PhRMA), Washington, DC |

Jacob T. Elberg
Chief, Health Care and Government Fraud Unit, United States Attorney’s Office, District of New Jersey, US Department of Justice, Newark, NJ
|

Gary F. Giampetruzzi, Esq.
Partner, Paul Hastings, Former Vice President and Assistant General Counsel, Head of Government Investigations, Pfizer Inc., New York, NY |

Virginia “Ginny” A. Gibson, Esq.
Partner, Hogan Lovells LLP, Former Executive Assistant U.S. Attorney, Eastern District of Pennsylvania, US Department of Justice, Philadelphia, PA |

Keith M. Korenchuk, JD, MPH
Partner, Arnold & Porter LLP, Washington, DC
|

Daniel A. Kracov, Esq.
Partner and Head, FDA and Healthcare Practice, Arnold & Porter, Washington, DC |

Dominique Laymand, Esq.
Senior Vice President, Chief Ethics and Compliance Officer, Ipsen, President, International Society of Healthcare Ethics and Compliance Professionals (ETHICS), Paris, France |

Michael K. Loucks, JD
Partner, Skadden Arps LLP; Former Acting United States Attorney, District of Massachusetts, United States Department of Justice, Washington, DC
|

Jason Mehta, JD
Assistant US Attorney, United States Attorney’s Office Middle District of Florida, US Department of Justice, Jacksonville, FL |

Kelly N. “Nikki” Reeves, MPA, JD
Partner, King & Spalding LLP, Washington, DC
|

Gregg Shapiro, JD
Assistant US Attorney, US Attorney’s Office, District of Massachusetts, US Department of Justice, Boston, MA |

Julie Ritchie Wagner, JD
Assistant General Counsel, PhRMA, Former Senior Counsel, Office of Counsel to the Inspector General, US Department of Health and Human Services, Washington, DC
|
|
|
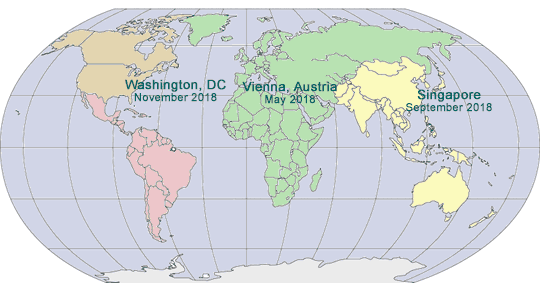
2017-2018 GLOBAL PHARMA COMPLIANCE CONGRESSES
|
|
ELEVENTH INTERNATIONAL PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
|
Sponsored by International Society of Healthcare Compliance Professionals (ETHICS)
Cosponsored by Pharmaceutical Compliance Forum (PCF)
May 15 - 17, 2017
Lisbon Marriott Hotel
Lisbon, Portugal
www.InternationalPharmaCongress.com
|
|
SEVENTH ASIA PACIFIC PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
|
Sponsored by Asia Pacific Healthcare Industry Compliance Team
Cosponsored by International Society of Healthcare Ethics and Compliance Professionals (ETHICS) and Pharmaceutical Compliance Forum (PCF)
September 13 - 15, 2017
InterContinental Shanghai, Shanghai, China
www.AsianPharmaCongress.com
|
|
EIGHTEENTH ANNUAL PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
|
A Hybrid Conference and Internet Event
Sponsored by Pharmaceutical Compliance Forum (PCF)
November 6 - 8, 2017
Mandarin Oriental
Washington, DC
www.PharmaCongress.com
|
|
INDIAN PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
|
International Society of Healthcare Compliance Professionals (ETHICS)
Cosponsored by International Society of Healthcare Compliance Professionals (ETHICS) and Pharmaceutical Compliance Forum (PCF)
Spring 2018
Mumbai, India
Coming Soon
|
|
|
PAST GLOBAL PHARMA COMPLIANCE CONGRESSES
|

2007 - Brussels

2008 - Paris

2009 - Rome

2010 - Berlin
|

2011 - Istanbul

2011 - Singapore

2012 - Budapest

2012 - Shanghai
|

2012 - São Paulo

2013 - Madrid

2013 Kuala Lumpur

2014 - Dubai
|

2014 - Mexico City

2014 - Shanghai

2015 - Manila

2016 - Warsaw
|
|
|
|
|
GRANTORS:
|
|
SILVER
|


|
|
BRONZE
|













|
|
CONTINUING EDUCATION CREDITS:
|
Accounting Professionals: Pending approval for NASBA CPE credits.
Attorney Credits: Pending Approval for PA MCLE credits.
Compliance Professionals: This education activity has been submitted to the Compliance Certification Board (CCB)® and is currently pending their review for approval of CCB CEUs.
Click here for more information.
|
|
MEDIA PARTNERS:
|



|
|
STAY CONNECTED
|


Tweet using #PharmaCongress
|
|
PHARMA CONGRESS IS
|

|
|
PARTICIPATION OPTIONS
|
|
TRADITIONAL ONSITE ATTENDANCE
|
Simply register, travel to the conference city and attend in person.
Pros: subject matter immersion; professional networking opportunities; faculty interaction

|
|
LIVE AND ARCHIVED WEBCAST PARTICIPATION
|
Watch the conference in live streaming video over the Internet and at your convenience at any time 24/7 for the six months following the event.
The archived conference includes speaker videos and coordinated PowerPoint presentations.
Pros: Live digital feed and 24/7 Internet access for next six months; Accessible in office, at home or anywhere worldwide with Internet access; Avoid travel expense and hassle; No time away from the office

|
|
|
|
|