|
|
|
Press Release: Save the Date for PCF Pharma Compliance Congress XIX, November 7 - 9, 2018, in Washington, DC
|
- Sponsored by the Pharmaceutical Compliance Forum
- November 7 - 9, 2018
- Onsite at the Mandarin Oriental, Washington, DC
- Online in Your Own Office or Home Live via the Internet with 24/7 Access for Six Months
- www.PharmaCongress.com
PRESS RELEASE
Phone: 800-503-7419
Email: registration@hcconferences.com
Website: www.PharmaCongress.com |
|
SPEAKER PRESENTATION PROPOSALS
|
Speaker/Presentation Proposals for the Nineteenth Pharma Congress May Be Submitted Through Our Online Form
- Click Here -
|
|
|
WASHINGTON, DC USA -- PHARMA UPDATE NEWS SERVICE™ -- DECEMBER 19, 2017: The Pharmaceutical Compliance Forum's Nineteenth Pharmaceutical and Medical Device Compliance Congress, www.PharmaCongress.com, to be held November 7 - 9, 2018 at the Mandarin Oriental in Washington, DC, today announced the conference dates and a call for presentation proposals. Registrants may attend the event in person or online (live webcast and archived for 6 months).
|
|
CO CHAIRS
|
Sujata T. Dayal, JD
Vice President, Health Care Compliance & Privacy, Pharmaceuticals Group, Johnson & Johnson, Titusville, NJ |

Jeffrey M. Kawalek, MBA
Senior Director, Ethics & Compliance, North America, Ipsen Biopharmaceuticals, Inc., Basking Ridge, NJ
|

Jennifer McGee, JD
Vice President and Chief Compliance Officer, Otsuka America Pharmaceutical, Inc., Rockville, MD |

Margaret Sparks, JD
Associate Vice President, North America Ethics and Business Integrity, Sanofi, US, Bridgewater, NJ
|
|
|
PARTICIPATION OPTIONS
|
|
TRADITIONAL ONSITE ATTENDANCE
|
|
 Simply register, travel to the conference city and attend in person.
Simply register, travel to the conference city and attend in person.
Pros: subject matter immersion; professional networking opportunities; faculty interaction.
|
|
LIVE AND ARCHIVED WEBCAST PARTICIPATION
|
|
 Watch the conference in live streaming video over the Internet and at your convenience at any time 24/7 for the six months following the event.
Watch the conference in live streaming video over the Internet and at your convenience at any time 24/7 for the six months following the event.
The archived conference includes speaker videos and coordinated PowerPoint presentations.
Pros: Live digital feed and 24/7 Internet access for next six months; Accessible in office, at home or anywhere worldwide with Internet access; Avoid travel expense and hassle; No time away from the office.
|
WEBCAST INTERFACE SAMPLE
Click here for a sample stream
|
|
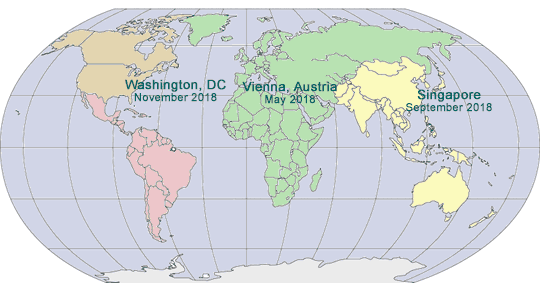
2018 GLOBAL PHARMA COMPLIANCE CONGRESSES
|
|
TWELFTH INTERNATIONAL PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
|
Sponsored by International Society of Healthcare Compliance Professionals (ETHICS)
Cosponsored by Pharmaceutical Compliance Forum (PCF)
Media Partners: Life Sciences Compliance Update
May 14 - 16, 2018
Hotel Savoyen
Vienna, Austria
www.International PharmaCongress.com
|
|
EIGHTH ASIA PACIFIC PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
|
Sponsored by Asia Pacific Healthcare Industry Compliance Team
Cosponsored by International Society of Healthcare Ethics and Compliance Professionals (ETHICS) and Pharmaceutical Compliance Forum (PCF)
Media Partners: Life Sciences Compliance Update
September 2018
Singapore
www.Asian PharmaCongress.com
|
|
INDIAN PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
|
International Society of Healthcare Compliance Professionals (ETHICS)
Cosponsored by International Society of Healthcare Compliance Professionals (ETHICS) and Pharmaceutical Compliance Forum (PCF)
Fall 2018
Mumbai, India
Coming Soon
|
|
NINETEENTH ANNUAL PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
|
A Hybrid Conference and Internet Event
Sponsored by Pharmaceutical Compliance Forum (PCF)
November 7 - 9, 2018
Mandarin Oriental
Washington, DC
www.PharmaCongress.com
|
|
|
|
|
|
SPONSORED BY
|

The Pharmaceutical Compliance Forum (PCF) is a coalition of senior compliance professionals and legal counsel from more than 70 of the largest research-based pharmaceutical manufacturers. The PCF was founded in early-1999 by compliance professionals from the pharmaceutical industry to promote effective corporate compliance programs.
|
FEATURED 2017
CONGRESS VIDEOS
|
|
Keynote: OIG Update
|

Mary E. Riordan, JD
Senior Counsel, Office of Counsel to the Inspector General, Office of Inspector General, HHS
Click here to view.
|
|
US DOJ Keynote: DOJ Evaluation of Corporate Compliance Program
|

Pablo Quiñones, JD
Chief, Strategy, Policy and Training Unit, Fraud Section, Criminal Division, USDOJ

Colin M. Huntley, JD
Assistant Director, Civil Division, Fraud Section, USDOJ

Gejaa T. Gobena, JD
Partner, Hogan Lovells, Former Deputy Chief, Fraud Section, Criminal Division, USDOJ, Former Trial Attorney, Civil Division, Fraud Section, USDOJ
Click here to view.
|
|
MEDIA PARTNERS:
|



|
|
STAY CONNECTED
|


Tweet using #PharmaCongress
|
|
PHARMA CONGRESS IS
|

|
|
|
|
|