|
|
|
Press Release: PCF Pharma Compliance Congress XIX Features Richard Bistrong, Chief Executive Officer, Front-Line Anti-Bribery LLC
|
- Sponsored by the Pharmaceutical Compliance Forum
- November 7 - 9, 2018
- Onsite at the Mandarin Oriental, Washington, DC
- Online in Your Own Office or Home Live via the Internet with 24/7 Access for Six Months
- Media Partners: Harvard Health Policy Review, Health Affairs and Life Sciences Compliance Update
- www.PharmaCongress.com
PRESS RELEASE
Phone: 800-503-7419
Email: registration@hcconferences.com
Website: www.PharmaCongress.com |
|
FEATURING KEYNOTE ADDRESS BY
|
 |
Richard Bistrong, MA, Chief Executive Officer, Front-Line Anti-Bribery LLC, Contributing Editor, FCPA Blog, Former Confidential Human Source (CHS) and Cooperating Witness, FBI and US DOJ, Former Cooperating Witness, City of London Police, HMRC and CPS, UK, New York, NY, USA
|
|
|
SPEAKER PRESENTATION PROPOSALS
|
Speaker/Presentation Proposals for the Nineteenth Pharma Congress May Be Submitted Through Our Online Form
- Click Here -
|
|
|
WASHINGTON, DC USA -- PHARMA UPDATE NEWS SERVICE™ -- JULY 26, 2018: The Pharmaceutical Compliance Forum's Nineteenth Pharmaceutical and Medical Device Compliance Congress, www.PharmaCongress.com, to be held November 7 - 9, 2018 at the Mandarin Oriental in Washington, DC, today announced the conference dates and a call for presentation proposals. Registrants may attend the event in person or online (live webcast and archived for 6 months).
|
|
ADDITIONAL KEYNOTE SPEAKERS
|

Thomas W. Abrams, RPh, MBA
Director, Office of Prescription Drug Promotion, US Food and Drug Administration, Silver Spring, MD |

Mary E. Riordan, Esq.
Senior Counsel, Office of Counsel to the Inspector General, Office of Inspector General, US Department of Health and Human Services, Washington, DC
|
|
|
CONGRESS CO CHAIRS
|

Michael R. Clarke, JD
Vice President, Corporate Compliance, Indivior Inc., Richmond, VA
(PCF Co-Chair) |
Sujata T. Dayal, JD
Vice President, Health Care Compliance & Privacy, Pharmaceuticals Group, Johnson & Johnson, Titusville, NJ
(PCF Secretary) |

Jeffrey M. Kawalek, MBA
Senior Director, Ethics & Compliance, North America, Ipsen Biopharmaceuticals, Inc., Basking Ridge, NJ
(PCF Chair)
|

Jennifer McGee, JD
Vice President and Chief Compliance Officer, Otsuka America Pharmaceutical, Inc., Rockville, MD
(PCF Treasurer) |

Margaret Sparks, JD
Associate Vice President, North America Ethics and Business Integrity, Sanofi US, Bridgewater, NJ
(PCF Co-Chair) |

Joe Zimmerman
Vice President and Chief Compliance Officer US, Ferring Pharmaceuticals, Parsippany, NJ
(PCF Co-Chair)
|
|
|
FEATURING AN INVITATION-ONLY CCO
ROUNDTABLE WITH
|

Julie Ritchie Wagner, JD
Assistant General Counsel, PhRMA; Former Senior Counsel, Office of Counsel to the Inspector General, US Department of Health and Human Services; Washington, DC |

Sally Q. Yates, JD
Partner, King and Spalding, Former Acting Attorney General, Former Deputy Attorney General, Former Assistant US Attorney, US Attorney's Office, Northern District of Georgia, US Department of Justice, Washington, DC
|
|
|
FEATURED FACULTY
|

Timothy Ayers, JD, MPH
Vice President, Chief Compliance Officer, Horizon Pharma PLC, Former VP, Chief Compliance Officer, Dendreon, Chicago, IL |

Ann Beasley, JD
Director, Navigant Consulting; Former Senior Vice President, Chief Compliance Officer, Biogen; Boston, MA |

John T. Bentivoglio, Esq.
Partner, Skadden Arps LLP; Former Special Counsel for Healthcare Fraud, and Chief Privacy Officer, US Department of Justice; Washington, DC
|

Kathleen Boozang
Dean and Professor of Law at Seton Hall Law, Newark, NJ |

Joseph Calarco
Chief Compliance Officer at Neos Therapeutics, Inc., Philadelphia, PA |

Christie Camelio
Vice President and US Healthcare Compliance Officer, Celgene Corporation, Summit, NJ
|

Brian Conner
Vice President, Head of Corporate Compliance, Strongbridge Biopharma plc, Trevose, PA |

Tom Costa, JD
Pharmaceutical Consultant, Aventis Board of Directors; Former Vice President, US Compliance & Ethics, Bristol-Myers Squibb; Washington, DC |

Jill Fallows-Macaluso, JD
Chief Compliance Officer and Vice President, Novo-Nordisk, Princeton, NJ
|

Margaret Feltz, JD, MA
Executive Director, Acting Chief Compliance Officer, Ethics & Compliance, Purdue Pharma L.P., Stamford, CT |

Gary F. Giampetruzzi, Esq.
Partner, Paul Hastings; Former Vice President and Assistant General Counsel, Head of Government Investigations, Pfizer Inc.; New York, NY |

Wendy C. Goldstein, JD
Partner, Health Care and Life Sciences Regulatory Practice, Cooley, LLP, New York, NY
|

Jonathon Kellerman
Executive Vice President, Global Chief Compliance Officer, Allergan PLC, Former Partner, PwC, Parsippany, NJ |

Amy Pawloski
Compliance Officer - Operations at Endo Pharmaceuticals, Former Global Lead, Compliance Risk Mitigation and Monitoring Strategy, Bristol-Myers Squibb; Philadelphia, PA |

Perri Pomper
Director - Ethics and Compliance Strategy & Education at Novo Nordisk
|

Kelly N. "Nikki" Reeves, MPA, JD
Partner, King & Spalding LLP, Washington, DC |

Michael Shaw, JD
Vice President and Compliance Officer, US Pharmaceuticals, GlaxoSmithKline; Former Senior Counsel, Office of Inspector General, US Department of Health and Human Services; Former Member, PCF Executive Committee; Philadelphia, PA |

Ann-Marie Tejcek, MA
Senior Director Ethics & Compliance, USA/Canada, Eli Lilly and Company, Indianapolis, IN
|

Donna White, CCEP
Vice President, Contracts & Compliance, Chiesi USA, Inc.; Former Sr. Director, Contracts and Compliance, Cornerstone Therapeutics; Cary, NC |

Seth B. Whitelaw, JD, LLM, SJD
President and Chief Executive Officer, Whitelaw Compliance Group, LLC; Editor, Life Science Compliance Update; Senior Fellow & Adjunct Professor, Life Sciences Compliance at Mitchell Hamline School of Law, West Chester, PA
|
|
|
PARTICIPATION OPTIONS
|
|
TRADITIONAL ONSITE ATTENDANCE
|
|
 Simply register, travel to the conference city and attend in person.
Simply register, travel to the conference city and attend in person.
Pros: subject matter immersion; professional networking opportunities; faculty interaction.
|
|
LIVE AND ARCHIVED WEBCAST PARTICIPATION
|
|
 Watch the conference in live streaming video over the Internet and at your convenience at any time 24/7 for the six months following the event.
Watch the conference in live streaming video over the Internet and at your convenience at any time 24/7 for the six months following the event.
The archived conference includes speaker videos and coordinated PowerPoint presentations.
Pros: Live digital feed and 24/7 Internet access for next six months; Accessible in office, at home or anywhere worldwide with Internet access; Avoid travel expense and hassle; No time away from the office.
|
WEBCAST INTERFACE SAMPLE
Click here for a sample stream
|
|
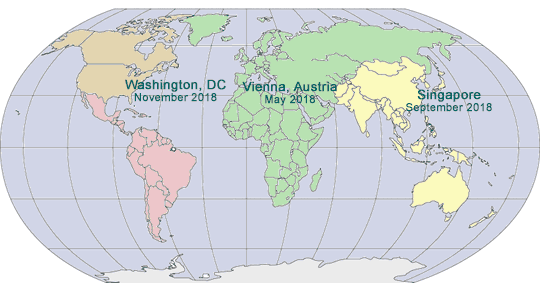
2018 GLOBAL PHARMA COMPLIANCE CONGRESSES
|
|
TWELFTH INTERNATIONAL PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
|
Sponsored by International Society of Healthcare Compliance Professionals (ETHICS)
Media Partners: Life Sciences Compliance Update
May 14 - 16, 2018
Hotel Savoyen
Vienna, Austria
www.International PharmaCongress.com
|
|
EIGHTH ASIA PACIFIC PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
|
Sponsored by Asia Pacific Healthcare Industry Compliance Team
Cosponsored by International Society of Healthcare Ethics and Compliance Professionals (ETHICS)
Media Partners: Life Sciences Compliance Update
September 10 - 12, 2018
Singapore
www.Asian PharmaCongress.com
|
|
NINETEENTH ANNUAL PHARMACEUTICAL AND
MEDICAL DEVICE COMPLIANCE CONGRESS
|
A Hybrid Conference and Internet Event
Sponsored by Pharmaceutical Compliance Forum (PCF)
November 7 - 9, 2018
Mandarin Oriental
Washington, DC
www.PharmaCongress.com
|
|
|
|
|
|
SPONSORED BY
|

The Pharmaceutical Compliance Forum (PCF) is a coalition of senior compliance professionals and legal counsel from more than 70 of the largest research-based pharmaceutical manufacturers. The PCF was founded in early-1999 by compliance professionals from the pharmaceutical industry to promote effective corporate compliance programs.
|
FEATURED 2017
CONGRESS VIDEOS
|
|
Keynote: OIG Update
|

Mary E. Riordan, JD
Senior Counsel, Office of Counsel to the Inspector General, Office of Inspector General, HHS
Click here to view.
|
|
US DOJ Keynote: DOJ Evaluation of Corporate Compliance Program
|

Pablo Quiñones, JD
Chief, Strategy, Policy and Training Unit, Fraud Section, Criminal Division, USDOJ

Colin M. Huntley, JD
Assistant Director, Civil Division, Fraud Section, USDOJ

Gejaa T. Gobena, JD
Partner, Hogan Lovells, Former Deputy Chief, Fraud Section, Criminal Division, USDOJ, Former Trial Attorney, Civil Division, Fraud Section, USDOJ
Click here to view.
|
|
GRANTORS:
|
|
SILVER
|



|
|
BRONZE
|








|
|
PHARMA CONGRESS IS
|

|
|
MEDIA PARTNERS:
|



|

 This site complies with the HONcode standard for trustworthy health information: This site complies with the HONcode standard for trustworthy health information:
verify here.


|

|
|
|
|
|