|
|
|
Press Release: PCF Pharma Compliance Congress XIX Agenda and Brochure Now Available
|
- Sponsored by the Pharmaceutical Compliance Forum
- November 7 - 9, 2018
- Onsite at the Mandarin Oriental, Washington, DC
- Online in Your Own Office or Home Live via the Internet with 24/7 Access for Six Months
- Media Partners: Harvard Health Policy Review, Health Affairs and Life Sciences Compliance Update
- www.PharmaCongress.com
PRESS RELEASE
Phone: 800-503-7419
Email: registration@hcconferences.com
Website: www.PharmaCongress.com |
EARLY BIRD REGISTRATION
- SAVE UP TO $400 -
|
Register by Friday, September 21, 2018 and
save up to $400.
Click here to register.
|
|
|
WASHINGTON, DC USA -- PHARMA UPDATE NEWS SERVICE™ -- SEPTEMBER 5, 2018: The Pharmaceutical Compliance Forum's Nineteenth Pharmaceutical and Medical Device Compliance Congress, www.PharmaCongress.com, will be held November 7 - 9, 2018 at the Mandarin Oriental in Washington, DC. Registrants may attend the event in person or online (live webcast and archived for 6 months).
|
|
KEYNOTE SPEAKERS
|

Thomas W. Abrams, RPh, MBA
Director, Office of Prescription Drug Promotion, US Food and Drug Administration, Silver Spring, MD |

Leslie Backschies, MA
Supervisor Special Agent and Unit Chief, International Corruption Unit, Federal Bureau of Investigation, Los Angeles, CA |

Richard Bistrong, MA
Chief Executive Officer, Front-Line Anti-Bribery LLC, Contributing Editor, FCPA Blog, Former Confidential Human Source (CHS) and Cooperating Witness, FBI and US DOJ, Former Cooperating Witness, City of London Police, HMRC and CPS, UK, New York, NY, USA
|

Hui Chen, JD
Ethics and Compliance Advocate and Consultant, HC Ethics LLC; Former Compliance Counsel Expert (Consultant), US Department of Justice; Former Assistant General Counsel, Pfizer, New York, NY |

Zachary A. Cunha, JD
Assistant US Attorney and Chief, Civil Division, US Attorney's Office, District of Rhode Island, US Department of Justice, Former AUSA, District of Massachusetts and Eastern District of New York, Providence, RI |

Susan Dentzer
President and Chief Executive Officer, NEHI, (The Network for Excellence in Health Innovation), Analyst on Health Policy, The NewsHour, Former Editor, Health Affairs, Washington, DC
|

Jacob Elberg, JD
Assistant US Attorney, US Attorney's Office, District of New Jersey, US Department of Justice, Newark, NJ |

Christopher B. Harwood, JD
Assistant US Attorney and Chief of the Civil Health Care Fraud Unit, US Attorney's Office, Southern District of New York, Former Assistant US. Attorney, US Attorney's Office for the District of Columbia, US Department of Justice, New York, NY |

Tarek Helou, Esq.
Assistant Chief, FCPA Unit, Fraud Section, Criminal Division, US Department of Justice, Washington, DC
|

Rachael Honig, JD
First Assistant US Attorney, US Attorney's Office for the District of New Jersey, Former Corporate Counsel, Litigation, Celgene, New York, NY |

Mary E. Riordan, Esq.
Senior Counsel, Office of Counsel to the Inspector General, Office of Inspector General, US Department of Health and Human Services, Washington, DC
|

Gregg Shapiro, JD
Assistant US Attorney, US Attorney's Office, District of Massachusetts, US Department of Justice, Boston, MA |

Sally Q. Yates, JD
Partner, King and Spalding, Former Acting Attorney General, Former Deputy Attorney General, Former Assistant US Attorney, US Attorney's Office, Northern District of Georgia, US Department of Justice, Washington, DC
|
|
|
AGENDA-AT-A-GLANCE
|
|
WEDNESDAY, NOVEMBER 7
|
|
|
|
FEATURING AN INVITATION-ONLY CCO ROUNDTABLE
|
- Hosted by PCF and PhRMA CCO Working Group; Featuring Hui Chen, JD, HC Ethics LLC
|
|
PRECONFERENCE SESSIONS
|
- Patient Support Programs: Risk and Risk Management Best Practices
- Practical Approaches to Handling Investigations
- Advanced Strategies in Auditing and Monitoring
- Asking the Right Risk Questions to Power your Advanced Analytics Strategy
|
|
PLENARY SESSIONS
|
- OIG Keynote Address
- FDA Keynote Address
- Risks Your Risk Assessment Might Miss
- Chief Compliance Officer Roundtable
|
|
THURSDAY, NOVEMBER 8
|
|
|
|
PLENARY SESSIONS
|
- Sally Yates Keynote Address
- Annual AUSA Roundtable
- Annual Qui Tam Roundtable
- DOJ, FBI and SEC FCPA Enforcement Update
- Patient Support Programs Roundtable
- Pharma and Device Pricing and Cost Containment
|
|
MINI SUMMITS
|
- Fostering a Culture of Ethics and Compliance
- Global Compliance Function Harmonization
- Third Party Lifecycle Management
- Annual Medical Device Compliance Roundtable
|
|
|
|
MINI SUMMITS, CONTINUED...
|
- Dissecting Recent CIAs and Review of Draft Model CIA
- Strategies to Measure Effectiveness, Monitor and Report
- Managing Promotional Programs and Patient Advocacy Groups
- Open Payments: Where Does the Sun Shine Now?
- Interactions with Specialty Pharmacy
- Is your Board Bored of your Compliance Dashboards?
- Next Generation Data Analytics Promotion with Medical Professionals: Avoiding White Coat Marketing
- Market Access Compliance Considerations
- Global Documentation Framework
- R&D: Hidden Risks beyond Clinical Trail Recruitment
- Compliance for Small to Mid-size Pharma and Device Companies
- Compliance Issues Associated with PBM Relationships
- Risk Assessments and Mitigation Plans
- "All in Compliance" -- Aligning with Your Business Partners
- Navigating the Murky Waters of Patient Programs
- Compliance for "Evolved" Products, including Aesthetics and Gene Therapy
- Best Practice in HCP/HCO Management
- Compliance Considerations in Value-based Contracting
- Risks and Opportunities related to Medical Affairs
- An Interactive Case Study in Effective Crisis Management
- Privacy and Cybersecurity Compliance Update, including GDPR
- The Compliance Training Revolution
|
|
FRIDAY, NOVEMBER 9
|
|
|
|
INDUSTRY-ONLY BEST PRACTICES ROUNDTABLE
|
- Featuring AUSA Gregg Shapiro, JD
|
|
|
FEATURED SESSION: ANNUAL CCO ROUNDTABLE WITH
|

Jill Fallows-Macaluso, JD
Chief Compliance Officer and Vice President, Novo-Nordisk, Princeton, NJ |

Indrani M. Lall Franchini, JD
Executive Vice President & Chief Compliance Officer, Alexion Pharmaceuticals, Inc., Former Assistant General Counsel, Pfizer, New York, NY |

Jonathon Kellerman
Executive Vice President, Global Chief Compliance Officer, Allergan PLC, Former Partner, PwC, Parsippany, NJ
|

Puja Leekha, JD
Vice President, Chief Compliance Officer and Corporate Counsel, Corporate Compliance, Lundbeck Pharmaceuticals LLC, Former Division Legal Counsel & Compliance Officer, Stryker Corporation, Deerfield, IL |

Lori Queisser
Sr VP and Global CCO, Teva, Former Sr. VP Global Compliance & Business Practices, Schering-Plough, Former VP & CCO, Eli Lilly, Former Co-chair, PCF, Horsham, PA |

Paul Silver
Principal, Regulatory & Compliance Life Sciences Leader, Deloitte Advisory, Deloitte, Atlanta, GA (Moderator)
|
|
|
FEATURED FACULTY
|

James Accumanno, JD
Director Risk, Monitoring and Auditing, Novo Nordisk, Former Associate Counsel, Bayer, Philadelphia, PA |

Anthony Alvizu, CPA, EnCE, CFE
Managing Director, Global Risk & Investigations Practice (GRIP), FTI Consulting, Chicago, IL |

Eliza L. Andonova, JD
Counsel, Hogan Lovells, Washington, DC |

Timothy Ayers, JD, MPH
Vice President, Chief Compliance Officer, Horizon Pharma PLC, Former VP, Chief Compliance Officer, Dendreon, Chicago, IL
|

Eric Baim, JD
Managing Director, Dovetail Consulting Group, LLC, Former VP, Head of US Compliance, Shire, Boston, MA |

Yogesh Bahl, CPA, MBA
Managing Director, Alix Partners, New York, NY |

Minna Bak, MBA
Senior Manager, Helio Health Group, New York, NY |

Jennifer Barbre, MA
Executive Director, Compliance, Spectrum Pharmaceuticals, Inc., Former Director, Compliance and Ethics Program, Avanir, Former Director, Commercial Compliance, Boehringer Ingelheim, Irvine, CA
|

Dennis K. Barnes, JD, MBA, CPA
Vice President, Global Governance, Risk and Compliance, Mayne Pharma; Former Vice President, Compliance and Risk Management, Global Compliance Officer, PAREXEL; Raleigh-Durham, NC |

Scott Bass, JD
Partner and Head, Global Life Sciences Team, Sidley Austin LLP, Chicago, IL |

Ann Beasley, JD
Director, Life Sciences, Governance, Risk & Compliance, Navigant, Former Senior Vice President, Chief Compliance Officer, Biogen, Boston, MA |

Thomas Beimers, JD
Partner, Hogan Lovells, Former Senior Counsel, Office of Counsel to the Inspector General, US DHHS, Former Special Assistant United States Attorney, US Attorney's Office, Eastern District of Michigan, Minneapolis, MN and Washington, DC
|

Wanda Bell, CHC
Director HCP Compliance, Nestle Skin Health, Former Director Commercial Compliance, Depomed, Former Director, Compliance, Elan, Former Compliance & Ethics Leader, AstraZeneca, Fort Worth, TX |

John T. Bentivoglio, Esq.
Partner, Skadden Arps LLP; Former Special Counsel for Healthcare Fraud, and Chief Privacy Officer, US Department of Justice; Washington, DC |

Brian A. Bohnenkamp, MHA, JD
Partner, FDA & Life Sciences Practice, King & Spalding LLP, Washington, DC |

Kathleen Boozang
Dean and Professor of Law at Seton Hall Law, Newark, NJ
|

Christine N. Bradshaw, JD
Vice President, Porzio Life Sciences, LLC, Principal, Porzio, Bromberg & Newman PC, Morristown, NJ |

Julianne Brierley
Director, Head of Compliance Learning, Novartis, New York, NY |

Katherine Buckley, MBA
Partner, Pharmaceutical and Life Sciences Advisory Practice, PwC, Philadelphia, PA |

Ed Buthusiem, JD
Managing Director, Berkeley Research Group, Adjunct Professor Of Law, Temple University, Former SVP, R&D Legal Operations & Vaccines, GSK, Devon, PA
|

Joseph Calarco
Chief Compliance Officer at Neos Therapeutics, Inc., Philadelphia, PA |

Christie Camelio
Vice President and US Healthcare Compliance Officer, Celgene, Former Head of Risk Management, Novartis, Summit, NJ |

Regina G. Cavaliere, JD
Principal, Regulatory Enforcement & Compliance, Life Sciences Sector, KPMG LLP, Former VP and CCO, Otsuka America, Short Hills, NJ |

Nicole Chandonnet, JD
Associate, Covington & Burling, Washington, DC
|

William Connelly, JD
Senior Counsel, Amgen; Former Staff Attorney, US DHHS, Washington, DC |

Brian Conner
Vice President, Head of Corporate Compliance, Strongbridge Biopharma plc, Trevose, PA |

Kellie Branson Combs, JD
Counsel, Ropes & Gray LLP, Washington, DC |

Thomas Costa, JD
Member, US Board of Directors, Sanofi; Former Vice President, US Compliance & Ethics, Bristol-Myers Squibb, Washington, DC
|

Clarissa Crain
Senior Manager, Life Sciences Advisory, Deloitte, Philadelphia, PA |

Paul Curtin
Vice President, Global Chief Compliance Officer, Intercept Pharmaceuticals; Former Compliance Officer Global Research and Development, Allergan f/k/a Actavis, New York, NY |

Jill Dailey, MBA, JD
Vice President & Chief Compliance Officer, Incyte, Former AG, Asia Pac Lead, Pfizer, Former Head, US Ethics and Compliance, Novartis, New York, NY |

Meenakshi Datta, JD
Partner and Global Co-leader, Healthcare Practice, Sidley Austin LLP, Chicago, IL
|

Mahnu V. Davar, JD
Partner, Arnold & Porter Kaye Scholer LLP, Lecturer, University of Pennsylvania Law School, Fellow, Salzburg Global Seminar, Washington, DC |

BJ D'Avella
Senior Manager, Life Sciences Regulatory and Operational Risk, Deloitte & Touche LLP, Atlanta, GA |

Sharon R. Delshad, JD
Director, Compliance and Legal Affairs, Nalpropion, Former Director, Compliance and Legal Affairs, Orexigen Therapeutics, San Diego, CA |

Gary Del Vecchio
Health Care CO Cardiovascular & Metabolism, Janssen, Former ED US Pharma Compliance & Ethics, Bristol-Myers Squibb, Former PCF Co-chair, New York, NY
|

Mark A. DeWyngaert, MBA, PhD
Managing Director, Life Sciences Regulatory and Operational Risk, Deloitte & Touche LLP, New York, NY |

Sarah diFrancesca, JD
Partner, Health Care and Life Sciences Regulatory Practice, Cooley, LLP, New York, NY |

Christian A. Dingler
Managing Consultant, Navigant Consulting, Richmond, VA |

Stefanie A. Doebler, JD
Of Counsel, Covington & Burling LLP, Washington, DC
|

Suzanne E. Durrell, JD
Partner, Whistleblower Law Collaborative, Taxpayers Against Fraud Education Fund, 2017 Whistleblower Lawyer of the Year, Boston, MA |

Michael Dusseau
Vice President, Compliance Operations, Allergan, Former Vice President, Compliance, Bayer, Former Divisional Compliance Officer, Merck, Madison, NJ |

Eileen Erdos
Principal, Forensic and Integrity Services, Ernst & Young LLP, Chicago, IL |

Margaret Feltz, JD, MA
Vice President, Ethics and Compliance, Purdue Pharma LP, Former PCF Co-chair, Stamford, CN
|

Alison Fethke, JD
Counsel, Ropes & Gray LLP, Former Division Counsel, Legal, Regulatory and Compliance, AbbVie, Chicago, IL |

Kellie Fidler, MBA, PMP
Director, Global Compliance Risk Mitigation & Monitoring Strategy, Bristol-Myers Squibb, Princeton Pike, NJ |

Francisco Ribeiro Filho, MBA
Senior Director & Head of US Compliance, Tesaro, Inc., Former Sr. Dir., Corporate Compliance, Biogen, Former Sr. Dir., International Markets, Sandoz, Boston, MA |

Paul Fishman, JD
Partner and Head, Crisis Management and Strategic Response Team, Arnold & Porter LLP, Former United States Attorney, District of New Jersey, Washington, DC
|

Paul Flanagan, JD
Assistant Professor of Law, Thomas R. Kline School of Law, Drexel University, Former Chief Compliance and Privacy Officer, 21st Century Oncology, West Chester, PA |

Sarah A. Franklin, JD
Partner, Covington & Burling LLP, Former Attorney, Federal Trade Commission, Washington, DC |

Alex Ganz, JD
Senior Manager of Compliance, Aegerion Pharmaceuticals, Former Compliance Manager, Shire and Vertex, Boston, MA |

Nereyda Garcia, JD
Global Heath, Ethics and Compliance, Alnylam Pharmaceuticals, Former Sr. Director, Compliance, Biogen Idec, Cambridge, MA
|

Gary F. Giampetruzzi, Esq.
Partner, Paul Hastings; Former Vice President and Assistant General Counsel, Head of Government Investigations, Pfizer Inc.; New York, NY |

Virginia "Ginny" A. Gibson, JD
Partner, Hogan Lovells LLP, Former First Assistant U.S. Attorney, Eastern District of Pennsylvania, US Department of Justice, Philadelphia, PA |

Jonathan Glazier, JD, MBA
Head, Legal Compliance, Philips, Former Sr. Dir., Corporate Compliance and Privacy Officer, Fresenius, Andover, MA |

Wendy C. Goldstein, JD
Partner, Health Care and Life Sciences Regulatory Practice, Cooley, LLP, New York, NY
|

Anthony Greco, MBA
Director, Pharmaceutical & Life Sciences Advisory Services, PwC, Philadelphia, PA |

Adam Greene, JD, MPH
Partner and Co-chair, Health Information & HIPAA Practice, Davis Wright Tremaine LLP, Former Senior Health Information Technology and Privacy Specialist, Office for Civil Rights, DHHS, Washington, DC |

Lillian S. Hardy, JD
Partner, Hogan Lovells LLP, Washington, DC |

Patricia J. Harned, PhD
Chief Executive Officer, ECI (Ethics and Compliance Initiative), Former President, Ethics Resource Center, Washington, DC
|

Kate Heinzelman, JD
Counsel, Sidley Austin LLP, Former Deputy GC, US DHHS, Former Special Assistant and Associate Counsel, President Barack Obama, Washington, DC |

Laura G. Hoey, JD
Co-leader, Government Enforcement and White Collar Group and Health Care and Life Sciences Industry Group, Ropes & Gray LLP, Boston, MA |

Christine Hood
Director, Learning and Development, Chiesi, Former District Sales Manager, Boehringer Ingelheim, Former Sales Trainer, Biovail, Apex, NC |

David Horowitz, JD
Partner, Hogan Lovells LLP, Former Deputy General Counsel, DHHS, Washington, DC
|

Casey J. Horton, CFE
Director, Life Sciences, Governance, Risk & Compliance, Navigant, Chicago, IL |

Becky Hsiao, JD
Senior Legal Manager, Americas Region, Diabetes Group, Medtronic, Los Angeles, CA |

Emily Huebener, MA
Forensic and Integrity Services, Ernst & Young LLP, Chicago, IL |

Erinn Hutchinson
Partner, Advisory Services, PwC, Philadelphia, PA
|

Michael Joachim, JD
Global Business Partner, Ethics & Business Integrity, Sanofi Genzyme, Cambridge, MA |

Elizabeth Jobes, JD
Head, Corporate Compliance & Legal Counsel, Spark, Former Senior VP & CCO, Auxilium, Former PCF Co-chair, Philadelphia, PA |

Darren R. Jones
Partner, Global Compliance Consulting Leader, Polaris Management, New York, NY |

Marci Juneau, MBA
Partner, Helio Health Group, Atlanta, GA
|

Kiley Smith Kelly, MBA
Partner, Forensic and Integrity Services, Ernst & Young LLP, Philadelphia, PA |

Keith M. Korenchuk, JD, MPH
Partner, Arnold & Porter LLP, Washington, DC |

Daniel A. Kracov, JD
Partner, Arnold & Porter Kaye Scholer LLP, Washington, DC |

Daryl Kreml, JD
VP & CCO, Sage, Former Head, US Compliance & Global Program Management, Biogen, Former Dir. & Sen. Managing Counsel, Int'l Compliance, Boston Scientific, Boston, MA
|

Bharathram Lakshmivarahan, MBA
Life Sciences Strategy & Operations Consultant, PwC, San Francisco, CA |

Marc Levine
Director of Compliance, Insightec, Former Compliance Officer, Stryker Corporation, Former Business Conduct Specialist, MAKO Surgical, Fort Lauderdale, FL |

Jonathan Levy, JD
Corporate Compliance Lead, Spark Therapeutics; Former Associate Director of Compliance and Ethics, US Pharmaceuticals, Bristol-Myers Squibb; Philadelphia, PA |

Jodi Lindsey, JD
Senior Director & Head of US Healthcare Compliance, Alkermes, Former Associate Director, Commercial Compliance, Biogen, Boston, MA
|

Richard Liner, JD
Senior Counsel, Compliance and Investigations, Bayer, Whippany, NJ |

Chad Londeree
Healthcare Industry Group Leader, SAI Global, Richmond, VA |

Pamela Lonzer, MSJ
Ass. Dir., Medical Operations, Compliance & Controls, Alexion, Former Ass. Dir. & US Medical Advisor, Global Compliance & Risk Management, Shire, Chicago, IL |

Seth H. Lundy, JD
Partner, King & Spalding, Washington, DC
|

Ali Lyons
Director, Ethics and Compliance, Aegerion, Former Director, Operations, Ethics and Compliance, Eli Lilly, Cambridge, MA |

Joe Mack, JD
Senior Compliance Counsel, Bayer, Former Assistant United States Attorney and Deputy Chief, Health Care and Government Fraud Unit, US Attorney's Office, District of New Jersey, New York, NY |

Colette G. Matzzie, JD
Partner, Phillips & Cohen LLP, Former Staff Attorney, Public Citizen Litigation Group, Former Trial Attorney, Tobacco Litigation Team, US Department of Justice, Washington, DC |

Dennis McCloskey, JD
Compliance Officer, Olympus Corporation of the Americas, Former Assistant District Attorney, Homicide Unit, Philadelphia District Attorney's Office, Philadelphia, PA
|

Vickie L. McCormick
Vice President, Janssen U.S. Commercial Health, Care Compliance & CIA Operations, Johnson & Johnson, Former Chief Compliance Officer, St. Jude Medical, Titusville, NJ |

Jennifer McGee, JD
Vice President and Chief Compliance Officer, Otsuka America Pharmaceutical, Inc., Rockville, MD |

Jean McKiernan, MBA
Director, Pharmaceutical and Life Sciences Advisory Practice, PwC, Chicago, IL |

Jason P. Mehta, JD
Partner, Bradley Arant Boult Cummings LLP, Former Assistant United States Attorney, US Attorney's Office for the Middle District of Florida, US Department of Justice, Tampa, FL
|

Kathleen Meriwether, JD
Principal, Fraud Investigation and Dispute Services, EY, Former Assistant United States Attorney, Eastern District of Pennsylvania, US Department of Justice, Philadelphia, PA |

Brian Miller, PhD
Dir. Compliance Training and Documentation, Otsuka, Former Senior Mgr, Learning Technologies, AstraZeneca, Former Ass. Dir. Learning Design & Technology, Merck, Princeton, NJ |

Vahan Minassian, JD
Director, US Promotional Monitoring Lead, Pfizer, Philadelphia, PA |

Melissa Murphy, MBA
Senior Compliance Specialist, Office of Ethics & Compliance, Medtronic, Minneapolis, MN
|

Katherine Cartwright Norris, MPA
Dir., Berkeley Research Group, Former Sr Dir., Compliance, NSF Health Sciences, Former Dir., Corporate Compliance, Spectranetics, Washington, DC |

Chrisoula Nikidis
Canadian Head of Compliance and Ethics Solutions, IQVIA; Former Executive Director, Ethics and Compliance, Innovative Medicines Canada; Former Industry Co-Chair Designate, APEC Biopharmaceutical Working Group on Ethics, Ottawa, Canada |

Adam Oakley
Director, Potomac River Partners, Arlington, VA |

Josh T. O'Harra, MS, JD
Assistant General Counsel, Eli Lilly and Company; Former Sr. Associate, King & Spalding, Washington, DC
|

Agatha O'Malley, MStPH, JD
Privacy Officer and Senior Counsel, Otsuka Pharmaceuticals (US), Former Director - Head of Privacy, Shire, Philadelphia, PA |

John Patrick Oroho, JD
Executive Vice President and Chief Strategy Officer, Porzio Life Sciences, LLC, Principal, Porzio, Bromberg & Newman PC, Morristown, NJ |

Amy Pawloski
Compliance Officer - Operations at Endo Pharmaceuticals, Former Global Lead, Compliance Risk Mitigation and Monitoring Strategy, Bristol-Myers Squibb; Philadelphia, PA |

Joe Philipose, JD
Vice President, US & Enterprise Compliance, Alexion, Former Director of Compliance, Salix, Former Director, PwC Life Sciences Consulting, Boston, MA
|

Roy Pollitt
Managing Director, Americas Head, Investigations, Exiger, Former Special Agent, Federal Bureau of Investigation, New York, NY |

Perri Pomper
Director - Ethics and Compliance Strategy & Education at Novo Nordisk |

Kelly N. "Nikki" Reeves, MPA, JD
Partner, King & Spalding LLP, Washington, DC |

Jeffrey Rosenbaum, MBA
Partner and Co-founder, Dovetail Consulting Group, LLC, Former SVP, Chief Compliance and Risk Officer, Shire, Boston, MA
|

David Ryan, JD
Vice President and Chief Compliance Officer, Epizyme, Former Vice President, Associate GC and CCO, Haemonetics, Former Vice President of Legal Affairs, Assistant General Counsel and CCO, AMAG Pharmaceuticals, Boston, MA |

Mark Scallon, MHA
Senior Principal, Polaris, IQVIA Global Compliance, Richmond, VA |

Philip Lo Scalzo, JD
Senior Vice President, Chief Compliance Officer, BioMarin; Former Assistant General Counsel, sanofi-aventis, Novato, CA |

Cheryl J. Scarboro, JD
Partner, Simpson Thacher & Bartlett, Former, Chief, FCPA Unit, Enforcement Division, US SEC, Washington, DC
|

Sue Seferian, JD
Health Care Compliance Officer, Johnson & Johnson, Titusville, NJ |

George Serafin, MS
National Health Care and Life Sciences Leader, Grant Thornton, LLP, Iselin, NJ |

Nicole Serena
Director, Patient Access, Bayer, Canada |

Patterson Shafer
Managing Director, Health Care and Life Sciences, Grant Thornton, Stamford, CT
|

Michael Shaw, JD
Vice President and Compliance Officer, US Pharmaceuticals, GlaxoSmithKline, Former Senior Counsel, Office of Inspector General, US DHHS, Former PCF Co-chair, Philadelphia, PA |

Laura (Munns) Skinner, MBA
Senior Manager, Life Sciences Advisory, Deloitte, Austin, TX |

John Smollen, MA, JD
Practice Professor of Law and Director, Center for Compliance and Ethics, Temple Law, Former EVP and CCO, Endo International, Philadelphia, PA |

Eugene F. Soltes, MBA, PhD
Jakurski Family Associate Professor of Business Administration, Harvard Business School, Author, Why They Do It: Inside the Mind of the White-Collar Criminal, Cambridge, MA
|

Oliver Steck
Principal, Life Sciences Regulatory and Operational Risk, Deloitte & Touche LLP, New York, NY |

Silvija A. Strikis, JD
Partner, Kellogg Hansen Todd, Figel & Frederick, Former Law Clerk, Justice Sandra Day O'Connor, US Supreme Court, Washington, DC |

Ravi Tayi, MD, MPH
Chief Medical Officer, US Ferring Pharmaceuticals; Former Vice President, Global Medical Affairs, Mallinckrodt; Former Vice President, Medical Affairs and Clinical Development, Cubist/Optimer, New York, NY |

Ann-Marie Tejcek, MA
Senior Director, Ethics & Compliance, Eli Lilly and Company, Indianapolis, IN
|

Yuet Ming Tham, JD
Partner and Global Head of White Collar Group, Sidley Austin, LLP, Former Asia Pacific Regional Compliance Director, Pfizer, Singapore and Hong Kong |

Bryan Timer, MA Business Analytics
Director, Data Analytics & Transparency, Merck, North Wales, PA |

Brian Van Hoy
Vice President, G&M Health LLC, Former Director, Ethics and Compliance, Eli Lilly, Bridgewater, NJ |

Jennifer Vondee, MPH
Manager, Pharmaceutical & Life Sciences Risk Consulting, PwC, Durham, NC
|

Julie Ritchie Wagner, JD
Assistant General Counsel, PhRMA; Former Senior Counsel, Office of Counsel to the Inspector General, US Department of Health and Human Services; Washington, DC |

Lisa Walkush, EMTM
Partner, Grant Thornton, LLP, Philadelphia, PA |

William Weinreb, JD
Partner, Quinn Emanuel, Former Acting United States Attorney and First Assistant US Attorney, Boston, Boston, MA |

Sarah Whipple, JD
Senior Corporate and Compliance Counsel, Akebia Therapeutics, Inc., Former VP, Global Compliance & Privacy Officer, Aegerion, Former CCO and Assoc. GC, AMAG, Boston, MA
|

Donna White
Vice President, Contracts & Compliance, Chiesi USA, Inc., Former Sr. Director, Contracts and Compliance, Cornerstone Therapeutics, Cary, NC |

Seth B. Whitelaw, JD, LLM, SJD
President and Chief Executive Officer, Whitelaw Compliance Group, LLC, Editor, Life Science Compliance Update, West Chester, PA |

Jonathan Wilkenfeld, MBA
Partner, Potomac River Partners, Washington, DC |

Kathryn "Katie" E. Winson
Senior Manager, US HCC Monitoring, Celgene Corporation, Summit, NJ
|
|
|
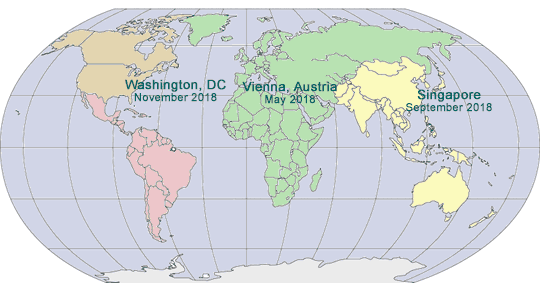
2018 GLOBAL PHARMA COMPLIANCE CONGRESSES
|
|
TWELFTH INTERNATIONAL PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
|
Sponsored by International Society of Healthcare Compliance Professionals (ETHICS)
Media Partners: Life Sciences Compliance Update
May 14 - 16, 2018
Hotel Savoyen
Vienna, Austria
www.International PharmaCongress.com
|
|
EIGHTH ASIA PACIFIC PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
|
Sponsored by Asia Pacific Healthcare Industry Compliance Team
Cosponsored by International Society of Healthcare Ethics and Compliance Professionals (ETHICS)
Media Partners: Life Sciences Compliance Update
September 10 - 12, 2018
Singapore
www.Asian PharmaCongress.com
|
|
NINETEENTH ANNUAL PHARMACEUTICAL AND
MEDICAL DEVICE COMPLIANCE CONGRESS
|
A Hybrid Conference and Internet Event
Sponsored by Pharmaceutical Compliance Forum (PCF)
November 7 - 9, 2018
Mandarin Oriental
Washington, DC
www.PharmaCongress.com
|
|
|
|
|
|
SPONSORED BY
|

The Pharmaceutical Compliance Forum (PCF) is a coalition of senior compliance professionals and legal counsel from more than 70 of the largest research-based pharmaceutical manufacturers. The PCF was founded in early-1999 by compliance professionals from the pharmaceutical industry to promote effective corporate compliance programs.
|
|
CONGRESS CO CHAIRS
|

Michael R. Clarke, JD
Vice President, Corporate Compliance, Indivior Inc., Richmond, VA
(PCF Co-Chair)
Sujata T. Dayal, JD
Vice President, Health Care Compliance & Privacy, Pharmaceuticals Group, Johnson & Johnson, Titusville, NJ
(PCF Secretary)

Jeffrey M. Kawalek, MBA
Senior Director, Ethics & Compliance, North America, Ipsen Biopharmaceuticals, Inc., Basking Ridge, NJ
(PCF Chair)

Jennifer McGee, JD
Vice President and Chief Compliance Officer, Otsuka America Pharmaceutical, Inc., Rockville, MD
(PCF Treasurer)

Margaret Sparks, JD
Associate Vice President, North America Ethics and Business Integrity, Sanofi US, Bridgewater, NJ
(PCF Co-Chair)

Joe Zimmerman
Vice President and Chief Compliance Officer US, Ferring Pharmaceuticals, Parsippany, NJ
(PCF Co-Chair)
|
|
GRANTORS:
|
|
GOLD
|
|
|
SILVER
|




|
|
BRONZE
|



















|
|
CONTINUING EDUCATION CREDITS
|
Accounting Professionals: Approved for up to 19.0 NASBA CPE Credits.
Attorneys: The Congress is currently pending approval to offer Pennsylvania MCLE Credit.
Compliance Professionals: This education activity has been submitted to the Compliance Certification Board (CCB)® and is currently pending their review for approval of CCB CEUs.
Click here for more information.
|
|
MEDIA PARTNERS:
|



|
|
PLANNING COMMITTEE
|
|
CO-CHAIRS
|
Michael R. Clarke, JD
Vice President, Corporate Compliance, Indivior Inc. (PCF Co-Chair)
Sujata T. Dayal, JD
Vice President, Health Care Compliance and Privacy, Pharmaceuticals Group, Johnson & Johnson (PCF Secretary)
Jeffrey M. Kawalek, MBA
Senior Director, Ethics and Compliance, North America, Ipsen Biopharmaceuticals, Inc. (PCF Chair)
Jennifer McGee, JD
Vice President and Chief Compliance Officer, Otsuka America Pharmaceutical, Inc. (PCF Treasurer)
Margaret Sparks, JD
Associate Vice President, North America Ethics and Business Integrity, Sanofi US (PCF Co-Chair)
Joe Zimmerman
Vice President and Chief Compliance Officer US, Ferring Pharmaceuticals (PCF Co-Chair)
|
|
MEMBERS
|
Kamil Ali-Jackson, JD
Co-Founder, Chief Legal Officer, Chief Compliance Officer and Corporate Secretary, Aclaris Therapeutics, Inc.
Michael Arbini
Global Marketing Communications Leader, Polaris Management
Timothy Ayers
Group Vice President, Chief Compliance Officer, Horizon Pharma PLC
Dennis K. Barnes, JD, CPA
Vice President, Global Governance, Risk and Compliance, Mayne Pharma
Scott Bass, JD
Partner and Head, Global Life Sciences Team, Sidley Austin LLP
Ann E. Beasley, JD
Director, Navigant Consulting
John T. Bentivoglio, JD
Partner, Skadden Arps LLP
Kathleen M. Boozang, JD, LLM
Dean and Professor of Law, Seton Hall University School of Law
Scott Boyance
Associate Director, Forensic and Integrity Services, EY
Joseph Calarco
Chief Compliance Officer, Neos Therapeutics
Christie Camelio
Vice President and US Healthcare Compliance Officer, Celgene Corporation
Thomas Costa, JD
Member, US Board of Directors, Sanofi; Former Vice President, US Compliance and Ethics, Bristol-Myers Squibb
Paul Curtin, JD, CFE
Vice President, Global Chief Compliance Officer, Intercept Pharmaceuticals
Jill Fallows-Macaluso, BSN, JD
Vice President and Chief Compliance Officer, Novo Nordisk Inc.
Margaret K. Feltz, MA, JD
Vice President, Ethics and Compliance, Purdue Pharma LP
Alison Fethke, JD
Counsel, Ropes & Gray LLP
Alex Ganz, JD
Senior Manager of Compliance, Aegerion Pharmaceuticals
Gary F. Giampetruzzi, JD
Partner, Paul Hastings
Virginia "Ginny" A. Gibson, JD
Partner, Hogan Lovells
Jonathan Glazier, JD, MBA
Head of Legal Compliance, Philips North America LLC
Wendy C. Goldstein, JD
Partner, Health Care and Life Sciences Regulatory Practice, Cooley, LLP
Ive Grgas, MS
Director, Healthcare Compliance, Puma Biotechnology
Bill Hrubes, MS
Vice President and Chief Compliance Officer, ACell, Inc.
Erinn Hutchinson
Partner, Advisory Services, PwC
Michael Joachim, JD
Compliance Business Partner, Sanofi Genzyme
Jonathon L. Kellerman
Executive Vice President, Global Chief Compliance Officer, Allergan PLC
Daniel A. Kracov, JD
Partner and Head, FDA and Healthcare Practice, Arnold & Porter Kaye Scholer, LLP
Holly Kramen, JD
Chief Compliance Officer and Privacy Officer, PolarityTE
Puja Leekha, JD
Vice President, Chief Compliance Officer and Corporate Counsel, Corporate Compliance Lundbeck
Gregory Moss, JD
Senior Vice President, Deputy General Counsel, Kadmon Corporation
Katherine Norris
Director, Berkeley Research Group
Neil F. O'Flaherty, JD
Partner, Baker Mckenzie
John Patrick Oroho, JD
Executive Vice President and Chief Strategy Officer, Porzio Life Sciences, LLC
Jennifer O'Toole
Events Manager, Americas, SAI Global
Amy Pawloski
Compliance Officer, Operations, Endo Pharmaceuticals
Lori Queisser
Senior Vice President and Global Chief Compliance Officer, Teva
David Ralston, JD, MPH
Senior Director, Associate General Counsel, Business Conduct, Gilead Sciences
Kelly N. "Nikki" Reeves, MPA, JD
Partner, King & Spalding
Jeffrey Rosenbaum, MBA
Partner, Dovetail Consulting Group, LLC
Michael Shaw, JD
Vice President and Compliance Officer, US Pharmaceuticals, GlaxoSmithKline
Paul J. Silver
Principal, Deloitte
Ann-Marie Tejcek
Senior Director, Ethics and Compliance, Eli Lilly and Company
Brian Van Hoy
Vice President, G&M Health LLC
Donna Wachman
National Industry Marketing Leader, Grant Thornton LLP
Julie Wagner, JD
Assistant General Counsel, PhRMA
Caroline West, JD
Global Chief Compliance Officer, Olympus Corporation
Donna White
Vice President, Contracts and Compliance, Chiesi USA, Inc.
Seth B. Whitelaw, JD, LLM, SJD
President and CEO, Whitelaw Compliance Group, LLC
|
|
STAY CONNECTED
|


Tweet using #PharmaCongress
|
|
PARTICIPATION OPTIONS
|
|
TRADITIONAL ONSITE ATTENDANCE
|
Simply register, travel to the conference city and attend in person.
Pros: subject matter immersion; professional networking opportunities; faculty interaction

|
|
LIVE AND ARCHIVED WEBCAST PARTICIPATION
|
Watch the conference in live streaming video over the Internet and at your convenience at any time 24/7 for the six months following the event.
The archived conference includes speaker videos and coordinated PowerPoint presentations.
Pros: Live digital feed and 24/7 Internet access for next six months; Accessible in office, at home or anywhere worldwide with Internet access; Avoid travel expense and hassle; No time away from the office


|
|
WEBCAST INTERFACE SAMPLE
|
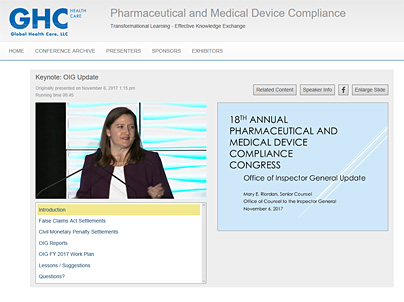
Click here for a sample stream
|
|
PAST GLOBAL PHARMA COMPLIANCE CONGRESSES
|
|

2007 - Brussels

2008 - Paris

2009 - Rome

2010 - Berlin

2011 - Istanbul

2011 - Singapore

2012 - Budapest

2012 - Shanghai

2012 - São Paulo

2013 - Madrid

2013 - Kuala Lumpur

2014 - Dubai

2014 - Mexico City

2014 - Shanghai

2015 - Manila

2016 - Warsaw

2017 - Lisbon

2018 - Vienna
|

|
|
|
|
|