|
|
|
Join Over 250 Attendees at the Virtual 14TH ETHICS Int'l Pharma & Med Device Ethics and Compliance Congress
|
- Virtual Online Video Event Live and 6 Months Access to Video Archive
- Sponsored by ETHICS, the International Society of Healthcare Compliance Professionals
- Media Partners: Policy & Medicine Compliance Update
- June 14-17, 2021
-
www.InternationalPharmaCongress.com
PRESS RELEASE
Phone: 800-503-8171
Email: registration@hcconferences.com
Website:
www.InternationalPharmaCongress.com
|
|
EARLY BIRD RATE
|
|
Click Here to register by May 14 for early bird discount.
|
|
NEW REGISTRATION RATES FOR A VIRTUAL AGE
|
Early bird registration discounts now available with special ETHICS/EFPIA/FSA/IFPMA/MedTech Europe/PCF rates. Rates are up to €1,000 less than major competing events.
- Click Here -
|
|
|
JOIN OVER 250 ATTENDEES FROM THE FOLLOWING ORGANIZATIONS
|
Abbvie
Abiomed
AdvaMed
Agence Francaise Anti-corruption
Align Technology
Alnylam
Amgen
Amicus Therapeutics
Argenx
Arthrex
Astellas
Avanir
Baker & McKenzie
Basel Institute on Governance
Bausch
Biologix
bioMerieux
Boehringer Ingelheim
Bristol-Meyers Squibb
Cergy Pontoise University
Clifford Chance
Comply Latam
ConvaTec
Covington & Burling
CSL Behring
Cutting Edge Information
Davis Wright Tremaine
Deloitte
DH Diagnostics
EFPIA
Ernst & Young
ESRI
ETHICS
European Multiple Sclerosis Platform
European Public Prosecutor's Office
|
F. Hoffmann-La Roche
Ferring
Front-Line Anti-Bribery
Getinge
GlaxoSmithKline
Guidehouse
Hillrom
Hogan Lovells
Hollister
Intercept
Int'l Association for the Study of Pain
IFPMA
Ipsen
IQVIA
Janssen-Cilag
Johnson & Johnson
King & Spalding
MCI
Medday
MedTech Europe
Medtronic
Menarini Group
Merck KGaA
Moderna
MorphoSys
NewBridge
Novartis
Novo Nordisk
NSN Law Firm
Olympus
Paris Centre for Quantum Computing
Paul Hastings
Perrigo
Pfizer
Pharmacie Gabou DIAWARA
|
Porzio Life Sciences
Principal Advisory
PtC Therapeutics
PwC
ResMed
Roche
Ropes & Gray
Sanofi
Sanofi Genzyme
Sciences Po
SeaGen
Seton Hall Law
Sidley Austin
Signature Litigation
Stryker
Sue Egan Assocs
Takeda
Terumo BCT
Teva
Trestle Compliance
UCB
UK SFO
United Therapeutics
University of Paris and University Hospital, Bobigny
US DOJ
US SEC
Verily Life Sciences
Viatris
Vifor
Vintura
Whispli
Zai Lab
Zimmer Biomet
|
|
|
PARIS, FRANCE -- HEALTHCARE UPDATE NEWS SERVICE™ -- APRIL 21, 2021: The Virtual Fourteenth International Pharmaceutical and Medical Device Ethics and Compliance Congress,
www.InternationalPharmaCongress.com, has been rescheduled to June 14 - 17, 2021, will take place in the form of online video broadcast which will be offered live and archived for 6 months following the live broadcast.
|
|
KEYNOTE SPEAKERS
|

Hani Abouhalka, MBA
Company Group Chairman, Medical Devices EMEA, Johnson & Johnson, New Brunswick, NJ, USA |

Gemma Aiolfi
Head of Compliance, Corporate Governance and Collective Action, Basel, Switzerland |

Pedro Carrascal, MBA
President, European Multiple Sclerosis Platform (EMSP), Chief Executive Officer, EME (Multiple Sclerosis Spain), Biscay MS Society and MS Basque Foundation, Board, Multiple Sclerosis International Federation (MSIF), Bilbao, Spain
|

Roxana Family, Thèse de Doctorat Droit
Vice-President, Chair in Law & Business Ethics and Program Director, Cergy Pontoise University, Cergy-Pontoise, France |

Robert I. Dodge, JD
Assistant Director, FCPA Unit, US Securities & Exchange Commission; Former Assistant U.S. Attorney, US Attorney's Office, Western District of Michigan; Former Assistant Section Chief, Environmental Defense Section, US Department of Justice, Washington, DC, USA |

David Fuhr, JD
Assistant Chief, FCPA Unit, Fraud Section, Criminal Division, US Department of Justice, Washington, DC, USA
|

Kirt Kraeuter, MGA
Senior Director, Corporate Compliance, Moderna; Former European Compliance Director, Otsuka; Former, Executive Director, Worldwide Compliance Committee & Monitoring, Bristol-Myers Squibb; Yardley, PA, USA |

Anna-Elisabeth Krause-Ablass, JD
European Delegated Prosecutor, European Public Prosecutor's Office; Former Specialized Public Prosecutor for White Collar Crime Frankfurt, Frankfurt, Germany |

Judy Krieg, MS, JD
Head of Fraud, Bribery and Corruption, Division B, UK Serious Fraud Office;
Former Chief Compliance Officer, Rolls-Royce plc; London, UK
|

Sally Molloy, JD
Chief, Strategy, Policy & Training Unit, Fraud Section; Former Assistant U.S. Attorney, US Attorney's Office, Northern District of Georgia, US Department of Justice; Washington, DC, USA |

Piergiorgio Pepe, MA
EU Law, President, Quantum Ethics, Ethics and Compliance Lecturer, SciencesPo; Former Compliance Director Western Europe & Canada, AbbVie; Former Director, Compliance & Ethics EMEA, Bristol-Myers Squibb; Paris, France |

N. Craig Smith
Chaired Professor of Ethics and Social Responsibility, Academic Director of ESRI (the INSEAD Ethics and Social Responsibility Initiative), and Programme Director for the Healthcare Compliance, Implementation Leadership Programme, INSEAD, Fontainebleau, France
|
|
|
|
FEATURING SENIOR ETHICS AND COMPLIANCE PROFESSIONALS ROUNDTABLE
|

Adam Dubow, JD
Senior Vice President, Chief Compliance and Ethics Officer, Bristol-Myers Squibb, Princeton, NJ, USA |

Mwana Lugogo, JD, MPP
Chief Ethics and Compliance Officer, Takeda Pharmaceuticals International GmBh, Zurich, Switzerland |

Lori Russell, JD
Chief Compliance Officer, bioMerieux, Lyon, France
|

Pascale Schmidt
Chief Compliance Officer and Chair, Corporate Sustainability Committee F. Hoffmann-La Roche Ltd, Basel, Switzerland |

Trudy Tan
Global Head Ethics, Risk & Compliance, Pharma, Novartis; Former Vice President Compliance, International, AstraZeneca; Basel, Switzerland |

Mariusz Witalis
Partner, Forensic & Integrity Services, EY, Warsaw, Poland
|
|
|
|
FEATURED FACULTY
|

Matt Abrahamson, MBA
Senior Compliance Analyst, DH Diagnostics LLC, a Danaher company, Buffalo Grove, IL, USA
|

Michaela Ahlberg, LLM, CCEP, CCEP-I
Senior Advisor, Getinge; Former Ethics & Compliance and Anti Bribery Corruption specialist, TheGreyZone, Göteborgsområdet, Sweden
|

Imelda Álvarez, LLB, MBA
Chief Executive Officer, Comply Latam, SC; Former Regional Integrity & Compliance Head Latin America and Canada, Novartis; Mexico City, Mexico
|

Ghadeer Al Yacoub, MSc
Regional Head Healthcare Compliance Europe, Middle East and Africa Medical Devices, Johnson & Johnson, Dubai, UAE
|

Bas Amesz, MSc
Partner, Vintura, Baarn, Utrecht, Netherlands
|

Michael Bartke, PhD
Director Ethics & Compliance, Alexion, Former Director Compliance Management, Daiichi Sankyo Europe GmbH, Strategic Committee, ETHICS, Munich, Germany
|

Ann Beasley, JD
Chief Compliance Officer, Zai Lab; Former Senior Vice President, Chief Compliance Officer, Biogen; Former Global Compliance Office, Novartis Pharma AG; Boston, MA, USA
|

Duygu Beyazo, LLB
Senior Associate, NSN Law Firm; Former Compliance Manager (Secondment), Business Conduct, Gilead Sciences; Istanbul, Turkey
|

Stefano Biondi, LLB, PhD
Compliance Manager and Group Data Protection Officer, Menarini Group, Florence, Italy
|

Richard Bistrong, MA
Chief Executive Officer, Front-Line Anti-Bribery LLC; Contributing Editor, FCPA Blog; Former Confidential Human Source (CHS) and Cooperating Witness, FBI and US DOJ; New York, NY, USA
|

Eric Bolesh
Chief Operating Officer, Cutting Edge Information, Raleigh-Durham, NC, USA
|

Julie Bonhomme
Legal & Compliance Director, European Federation of Pharmaceutical Industries and Associations (EFPIA), Brussels, Belgium
|

Amanda Bunten, PhD
Chartered Health Psychologist; Director of Behavioral Ethics, GlaxoSmithKline;Former Principal Behavioural Insights Advisor,Public Health England, London, UK
|

Keith Burn
Global Investigations Director, Ipsen; Former Associate Investigator, Parliamentary and Health Service Ombudsman, NHS; Former Detective Constable, London Metropolitan Police; Slough, UK
|

Masha Chestukhin, MSJ
Associate Director, Compliance Officer R&D, IA, FMV, Sanofi Genzyme; Former Senior Manager NA Compliance, Sanofi; Jamaica Plain, MA, USA
|

Kishwar Chishty, ACA
Partner Risk Advisory Life Sciences, Deloitte Switzerland, Basel, Switzerland
|

Ana Christian, JD
Senior Director, Assistant General Counsel, Avanir Pharmaceuticals; Former Corporate Counsel, NantHealth,Los Angeles, CA, USA
|

Michael R. Clarke, JD, CCEP
Vice President, Global Chief Compliance Officer, ConvaTec; Former Vice President, Corporate Compliance, Indivior; Former Vice President, Ethics & Compliance, Americas, Actavis; Bridgewater, NJ, USA
|

Zachary N. Coseglia, JD
Managing Principal and Head of Innovation, R&G Insights Lab, Ropes & Gray, LLP, Boston, MA, USA
|

Rika De Ville
Vice President Global Compliance & Privacy, Data Protection Officer, Perrigo Company plc Nazareth, Belgium
|

Stefanie Deronne, JD
Legal Counsel, Argenx; Former Senior Corporate Legal Counsel R&D - DPO, Ablynx; Ghent, Belgium
|

Anisa Dhalla
Global Chief Compliance Officer, UCB, Inc., Atlanta, GA, USA
|

Holger Diener
Healthcare Compliance Officer, Janssen-Cilag GmbH, Johnson & Johnson; Former Managing Director, Association of Voluntary Self-Regulation for the Pharmaceutical Industry (“FSA”), Berlin, Germany
|

Peter Dieners, Esq.
Regional Managing Partner (Germany) and Head, Global Healthcare and Life Sciences Group, Clifford Chance; Member, Legal Affairs Focus Group and Compliance Network, MedTech Europe; Member, Legal Affairs Committee and Healthcare Compliance Committee, BVMed, Düsseldorf, Germany
|

Kalwant Dhindsa, CIPP/E, CCEP
Compliance Director, Convatec; Former Senior Corporate Compliance Counsel, Bayer; Reading, UK
|

Laetitia Ducroquet
Vice-President, Global Business Ethics, Ipsen, Boulogne Billancourt, France
|

Julien Durand, MBA, PhD
Vice President, Head of Transformation, Global Ethics & Compliance, Takeda Chair, Future Health Technologies & Bioethics Working Groups, IFPMA; Former Executive Director Compliance & Ethics, Amgen; Former Chief Privacy Officer and Global Technology Compliance Head, AstraZeneca; Zürich, Switzerland
|

Sue Egan
Director and Principal Consultant, Sue Egan Associates; Former Vice President Compliance, AstraZeneca; Great Missenden, Buckinghamshire, UK
|

Signe Elbæk, JD, LLM
Former Group Chief Compliance Officer, Coloplast; Former Vice Chair, Ethics and Compliance Committee and Member of the Code Committee, MedTech Europe; Former Compliance Manager, Attorney, Dako Denmark A/S; Copenhagen, Denmark
|

Jacob Elberg, JD
Associate Professor, Seton Hall University School of Law; Former Chief, Health Care & Government Fraud Unit, and Assistant US Attorney US Attorney's Office, District of New Jersey; Newark, NJ, USA
|

Robert Ennis, JD, LLM
Vice President and Lead Compliance Counsel, Pfizer, New York, NY
|

Martin Eschbach, JD
Head Ethics, Risk and Compliance and Legal Patient Engagement, Novartis International AG, Baden-Württemberg, Germany
|

Steffen Esche
Director, Consulting, Risk & Regulatory, PwC, Frankfurt, Germany
|

J. Mark Farrar, MSJ, CPA, CFE, CFF, CIPP/US
Partner, Life Sciences Governance, Risk Management and Compliance, Guidehouse; Former Interim Chief Global Compliance Officer, Beckman Coulter, Atlanta, GA
|

Alex Fell
Head Ethics, Compliance and DPO International, Amicus Therapeutics; Former Vice President, Global Ethics and Compliance, Head of Strategy, Planning and Operations, GSK; London, UK
|

George Fife
Partner, Forensic & Integrity Services, EY, Former Executive Director, Compliance & Ethics, Bristol-Myers Squibb, Paris, France
|

Sylvie Gallage-Alwis, DEA, LLM
Partner, Signature Litigation Avocat à la Cour Solicitor in England & Wales Committees Vice Chair, International Association of Defense Counsel, Paris, France
|

Abhiroop Gandhi
Trust and Compliance Officer, Verily Life Sciences (an Alphabet company), Former Vice President, Corporate Compliance, Mallinckrodt, San Francisco, CA, USA
|

Gary F. Giampetruzzi, Esq.
Partner, Paul Hastings, Former Vice President and Assistant General Counsel, Head of Government Investigations, Pfizer Inc., New York, NY, USA
|

Gejaa T. Gobena, JD
Partner, Hogan Lovells; Former Deputy Chief, Fraud Section, Criminal Division; Former Trial Attorney, Civil Division, Fraud Section, US Department of Justice, Washington, DC
|

Tom Gregory
Partner, Fraud Investigation & Dispute Services, Ernst & Young, LLP, Atlanta, GA
|

Ulf H. Grundmann
Partner, FDA and Life Sciences, King & Spalding LLP, Frankfurt am Main, Germany
|

Ethan Gumpert
Global Ethics, Compliance & Governance, Advanced Medical Technology Association (AdvaMed), Washington, DC, USA
|

Joseph W. Henein, PharmD
President and Chief Executive Officer, NewBridge Pharmaceuticals, Dubai, UAE
|

Bella Rafael Hovhannisyan
Acting Chief Compliance Officer, Business Integrity Lead, Intercontinental, CSL Bering; Former Compliance & Ethics Lead, Spain & Portugal, Russia & Turkey, Bristol-Myers Squibb; Berne, Switzerland
|

Mirgen Jaku, MA
Lead of Market Readiness, Digital Surgery, EMEA, Johnson & Johnson, Hamburg, Germany
|

Franziska Janorschke
Global Head, Business Practices Office, Novartis International AG, Basel, Switzerland
|

Els Janssens, LLM
Counsel, Baker & McKenzie; Former Legal Adviser, European Medicines Agency; Former Senior Legal Counsel, Johnson & Johnson; Brussels, Belgium
|

Larisa K. Jasnic, LLB, LLM
Head of Ethics & Compliance, International, Alnylam Pharmaceuticals; Former Regional Compliance Officer, Region Europe, Novartis; Zug, Switzerland
|

Chelsea M. Keeton, JD
Director and Senior Counsel, Global Compliance Investigations, Zimmer Biomet; Former Group Ethics & Compliance Counsel, VEON, Amsterdam, Netherlands
|

Iordanis Kerenidis, PhD
Director, Paris Centre for Quantum Computing (PCQC) CNRS Senior Researcher (DR2), Algorithms and Complexity Group IRIF, University Paris Diderot, Paris, France
|

Elisabeth Kohoutek
Senior Associate, FDA and Life Sciences, King & Spalding LLP, Frankfurt am Main, Germany
|

Keith Korenchuk, MPH, JD
Vice President and Chief Compliance Officer, DH Diagnostics, a Danaher Company, Chevy Chase, MD, USA
|

Adem Koyuncu, MD, PhD (Law)
Partner and Chair, Food, Drug & Device Practice Group, Covington; Brussels, Belgium & Frankfurt, Germany
|

Aline Lautenberg, LLM
General Counsel, Director Legal & Compliance, MedTech Europe, Brussels, Belgium
|

Lei Li, LLM
Managing Partner, Beijing and Shanghai Offices, Sidley Austin; Former Third Secretary, Ministry of Commmerce, People's Republic of China, Beijing, China; Beijing, China
|

Abdul Luheshi, MBA
Independent Consultant, Ethics and Compliance; Former Compliance Officer, International Markets, Myriad Genetics; Former Executive Director, Global Operations, Ethics & Compliance, EMEA, Astellas;
London, UK
|

Rosa Magistri, CIPP/E
Head of Legal and Compliance Europe, SeaGen International GmbH; Former Regional Director Office of Ethics and Compliance, Western Europe & Canada, Region South & Israel, AbbVie; Former Associate Compliance Director, Shire; Zug, Switzerland
|

Sylvain Mansotte
Co-Founder and Chief Executive Officer, Whispli, Sydney, New South Wales, Australia
|

Anthony McQuillan, LLB
Vice President Legal & Compliance EMEA, Medtronic International; Former Senior Attorney EMEA, Hewlett-Packard; London, UK
|

Sofie Melis
Director HR and Ethics & Compliance, International Federation of Pharmaceutical Manufacturers and Associations (IFPMA), Geneva, Switzerland
|

Paul J. Melling, JD
Founding Partner, Baker & McKenzie, CIS, Limited, Moscow, Russia
|

Genevieve Michaux, JD
Partner, King & Spalding, Brussels, Belgium
|

Vahan Minassian, JD
Digital Compliance Counsel, Compliance Division, Global Programs, Pfizer, Philadelphia, PA, USA
|

Philippa Montgomerie, CPE, LLB
Vice President EMEA Compliance and Global Programs, Medtronic Limited, Watford, UK
|

Arthur Muratyan, Esq.
Secretary General, International Society of HealthCare, Ethics and Compliance Professionals (ETHICS), Chair, MedTech Compliance Panel; Former VP-Head of Legal Corporate and Global Compliance Officer, Sanofi
|

Laura Nassar, PharmD
Vice President, Head of Ethics & Business Integrity, AEME Region, Sanofi; Former Head of Compliance Middle East, Roche Pharmaceuticals; Former Regional Pharma HCC Officer Emerging Markets, Johnson & Johnson, Beirut, Lebanon
|

Gina Nese, JD
Vice President Global Compliance and Ethics Officer, Align Technology; Former Chief Compliance Officer, Advanced Sterilization Products; Bellevue, WA, USA
|

Chrisoula Nikidis, LL.L.
Head of Ethics and Compliance, Takeda Canada; Former Vice President, Ethics, Integrity, & Governance, Innovative Medicines Canada, Toronto, Canada
|

Lauri G. Opar
Head, Enterprise Risk Management & Risk Analytics, Global Ethics & Compliance, Glaxosmithkline, Brentford, Middlesex, UK
|

John Patrick Oroho, JD
Executive VP & Chief Strategy Officer, Porzio Life Sciences, LLC; Principal, Porzio Bromberg & Newman, PC, Morristown, NJ, USA
|

Tara Palesh, MS
Senior Director, Compliance Analytics Lead, Pfizer, New York, NY, USA
|

Giota Papamarkou
Vice-President, Business Ethics Global, France North America & Global Monitoring, Ipsen; Former EMEA Compliance and Ethics Manager, Bristol-Myers Squibb, Paris, France
|

Carrie (Ashcom) Pennington, MBA, PhD
Vice President, Global Compliance Monitoring & Internal Controls, Zimmer Biomet; Former Global Compliance Manager, Books & Records, GE Healthcare, Warsaw, IN, USA
|

Oscar Perdomo
Director, Pharmaceutical & Life Sciences, Advisory Services, PwC Switzerland, Zurich, Switzerland
|

Mario Prohasky
Principal, EMEA Compliance Consulting Lead, IQVIA, London, UK
|

Ariadna Quesada, MSJ
Compliance Director Europe and MEATI, Hillrom; Former Law, Ethics and Compliance Officer, The Netherlands, AbbVie; Former Compliance Manager International, MicroPort Orthopedics; Amsterdam, Netherlands
|

Amanda N. Raad, JD
Partner and Co-Chair of Global Anti-Corruption and International Risk Practice and R&G Insights Lab, Ropes & Gray, LLP, London, UK
|

Nadege Rochel, CCEP-I
Global Compliance Manager, Holister Incorporated, Milan, Italy
|

David Rodin, PhD
Founder and Chief Executive Officer, Principia Advisory, Former Co-Director, Institute for Ethics, Law, and Armed Conflict, Oxford University, Trelex, Vaud, Switzerland
|

Christian-Claus Roth
Global Head Scientific Engagement Governance, Novartis; Co-president, International Pharmaceutical Congress Advisory Association (IPCAA); Member, Ethics and Business Integrity Committee, IFPMA, Basel, Switzerland
|

Giuseppe Saporito, LLM
Global Legal & Regulatory Expert, IQVIA, Milan, Italy
|

Pascale Schmidt
Chief Compliance Officer and Chair, Corporate Sustainability Committee, F. Hoffmann-La Roche Ltd, Basel, Switzerland
|

Brian P. Sharkey
Director, US Commercial Compliance & Ethics, Teva Pharmaceuticals, Parsippany, New Jersey, USA
|

Leonardo Silva, LLM
Vice President, Global Chief Compliance & Privacy Officer, Ferring Pharmaceuticals; Former Head of Compliance, Acino Group, Acino International AG; Former Compliance Director, Emerging Markets, Takeda, Lausanne, Switzerland
|

Paul Silver
Principal, Regulatory & Compliance Life Sciences Leader, Deloitte Advisory, Atlanta, GA, USA
|

Sapan Singh, MBA
Senior Director Compliance, Stryker Corporation, Mahwah, NJ, USA
|

Cerstin Steindorf
Global Account Director Healthcare, MCI, Geneva, Switzerland
|

Robert Stephenson
Director, PwC UK, London, UK
|

Tiffany Tang
Associate Principal, Global Consulting, IQVIA; Former Senior Manager, Ethics and Compliance, Novo Nordisk; New York, NY
|

Kathy Tench, MSc
Risk Advisory director & Life Sciences Ethics/Compliance Lead, Deloitte Switzerland, Former Senior Director of Compliance, Optimer Pharmaceuticals, Basil, Switzerland
|

Heidi Teresi
Director, US Ethics & Compliance, Bausch Health; Former Lead, Education and Communication, US Compliance and Ethics, Bristol-Myers Squibb, Princeton, NJ
|

Madina Torchinova, JD
Vice President, Head Global Compliance, MorphoSys; Former Regional Head of Compliance, Central & Eastern Europe, Middle East & Africa, and Global OTC, Sandoz; Munich, Germany
|

Nancy S. Travis, MS
Vice President, International Compliance and Governance, Advanced Medical Technology Association (ADvaMed), Washington, DC, USA
|

Steve Vincze, JD, LLM, MBA
President and Chief Executive Officer, Trestle Compliance; SVP & US CCO, Cycle Pharmaceuticals; Former VP, Ethics & Compliance Officer, TAP Pharmaceuticals, Boston, MA
|

Samar Wakim, PharmD
Ethics & Compliance Head MEA, Innovative Medicines, Novartis Pharma Services AG, Dubai, UAE
|

Caroline H. West, JD
Former Global Chief Compliance Officer, Olympus Corporation; Former Senior Vice President, Chief Compliance and Risk Officer, Shire, Philadelphia, PA, USA
|

Vanessa Westphal, JD, MS
Head of Compliance Center of Excellence Programs & Support, Merck KGaA, Darmstadt, Frankfurt Am Main Area, Germany
|

Mariusz Witalis
Partner, Forensic & Integrity Services, EY, Warsaw, Poland
|

Elisabethann Wright, LLB
Partner, Hogan Lovells; Former, Senior Legal Officer and Hearing Officer, EFTA Surveillance Authority, Brussels, Belgium
|
|
|
|
|
|
|
CO CHAIRS
|

Anne-Sophie Bricca, MA, DEA International Law, DES European law
Deputy General Counsel & Senior Director Legal Affairs & Compliance, Terumo BCT; Chair, Ethics and Compliance Group and seats at the Code Committee, MedTech Europe; Brussels, Belgium
|

Stephen Nguyen Duc
Vice-President, Global Head of Human Resources and Ethics & Compliance, Medday Pharmaceuticals; Board Member, Strategic Committee International Society of Healthcare Ethics and Compliance Professionals (ETHICS); Paris, France
|

Dominique Laymand, Esq.
Executive Vice President, Chief Ethics & Social Responsibility Officer, Ipsen; Honorary President, International Society of Healthcare Ethics and Compliance Professionals (ETHICS); Paris, France
|

Roeland Van Aelst
Regional Lead Third Party Intermediary Ethics & Compliance EMEA, Johnson & Johnson, President, International Society of Healthcare Ethics and Compliance Professionals (ETHICS),Chairman, MedTech Europe Code Committee, Brussels, Belgium
|
|
SPONSORED BY:
|

|
|
GRANTORS
|
|
GOLD
|

|
|
SILVER
|


|
|
BRONZE
|







|
|
MEDIA PARTNER:
|

|
|
STAY CONNECTED
|


Tweet using #PharmaCongress
|
|
CONGRESS HIGHLIGHTS
|
- Live and curated sessions streamed directly to you through our easy to navigate advanced virtual broadcast platform
- New content, optimized for online learning, focused on new regulatory developments and managing ethics and compliance programs during the COVID 19 pandemic
- Engage with faculty through polling, Q & A sessions, chat and direct video messaging
- Network in real time with other attendees through direct attendee to attendee and group video messaging
- Access to an extensive video archive of past Congress presentations providing a context for the evolution of the compliance professions and the legal and regulatory setting
- Virtual exhibit hall featuring innovative solutions, products and services with opportunity to directly contact exhibitors for further information
|
|
|
TUITION SCHOLARSHIPS
|
The International Congress is now offering a limited number of partial and full Tuition Scholarships to qualifying representatives of government, consumer advocate organizations, academics, students, representatives of health services research organizations, and those who have lost their jobs as a result of the COVID-19 pandemic.
Click here for more information.
|
|
REGISTRATION DISCOUNTS AVAILABLE FOR MEMBERS OF
|






|
|
|
|
PAST GLOBAL PHARMA COMPLIANCE CONGRESSES
|

2007 - Brussels

2008 - Paris

2009 - Rome

2010 - Berlin

2011 - Istanbul

2011 - Singapore

2012 - Budapest

2012 - Shanghai

2012 - São Paulo

2013 - Madrid

2013 - Kuala Lumpur

2014 - Dubai

2014 - Mexico City

2014 - Shanghai

2015 - Manila

2016 - Warsaw

2017 - Lisbon

2018 - Vienna

2019 - Athens
|
|
|
FEATURING THE INTERNATIONAL CONGRESS ADVANCED VIRTUAL STREAMING PLATFORM
|

|
|
2021 GLOBAL PHARMA/MED DEVICE ETHICS & COMPLIANCE CONGRESSES
|
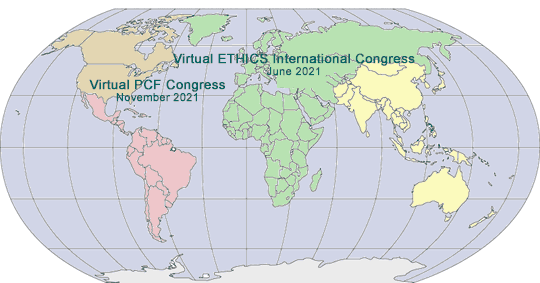
|
VIRTUAL FOURTEENTH INTERNATIONAL PHARMACEUTICAL AND MEDICAL DEVICE ETHICS & COMPLIANCE CONGRESS
|
Virtual Online Video Event Live and Archived
Sponsored by International Society of Healthcare Compliance Professionals (ETHICS)
Media Partner:
Policy & Medicine Compliance Update
June 14-17, 2021
www.International PharmaCongress.com
|
|
VIRTUAL TWENTY-SECOND ANNUAL PHARMACEUTICAL AND MEDICAL DEVICE ETHICS & COMPLIANCE CONGRESS
|
Virtual Online Video Event Live and Archived
Sponsored by Pharmaceutical Compliance Forum
Media Partners:
Harvard Health Policy Review, Health Affairs and
Policy & Medicine Compliance Update
November, 2021
www.PharmaCongress.com
|
|
|
|
CONGRESS EXHIBIT & SPONSORSHIP INFORMATION
|
|
For sponsorship and exhibit information, visit or contact Suzanne Tyler, Exhibit Manager, at (206) 244-4861 phone, (206) 319-5303 fax, or
exhibits@hcconferences.com.
|
|
FOR E-MAIL ADDRESS CHANGE, ADD OR DELETE REQUESTS
|
For changes or additions, please email your request to: listmgr@PharmaUpdateNewsService.com.
For removal of your e-mail address, please click the link below for "SafeUnsubscribe" to automatically remove your address from the list.
|
|
|
|

